Page 370 - Mechanism and Theory in Organic Chemistry
P. 370
1,2-Elimination Reactions 357
is formed, then the /3 proton lost to solvent in the second step should come with
equal probability from either the same or the opposite side of the plane as the
original leaving group. This prediction is in accord with experimental results.67
If, however, an intimate ion pair is formed and the leaving group rather than the
-.
solvent acts as the base, then syn elimination should resultJhqxedictionhas
For
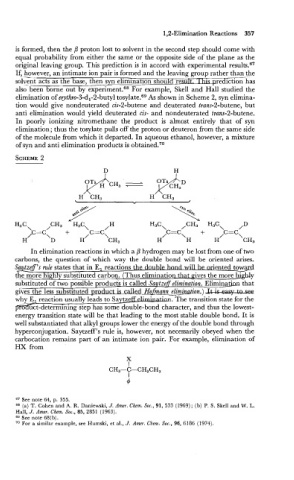
also- been borne out by e~periment.~~ example, Skell and Hall studied the
elimination of erythro-3:d,-2-butyl to~ylate.~~ As shown in Scheme 2, syn elimina-
tion would give nondeuterated cis-2-butene and deuterated trans-2-butene, but
anti elimination would yield deuterated cis- and nondeuterated tram-2-butene.
In poorly ionizing nitromethane the product is almost entirely that of syn
elimination; thus the tosylate pulls off the proton or deuteron from the same side
of the molecule from which it departed. In aqueous ethanol, however, a mixture
of syn and anti elimination products is obtained.70
H CH3 H CH,
In elimination reactions in which a /3 hydrogen may be lost from one of two
carbons, the question of which way the double bond will be oriented arises.
S'tzef's rule states that in E, reactions the double h d will be oriented towgd
the more highly substituted carbon. (Thus elimination that gives the more 1iigMy
substituted of two possible is called ~aqtzeff elimina&n. Elimm that
gives the less substit.uted ~mcalled Hofmann elim&tion.) I+is9asJI-t4-seg
why E, reaction usually leads to Saytzeff eliminatinn. The transition state for the
-
vuct-determining step has some double-bond character, and thus the lowest-
energy transition state will be that leading to the most stable double bond. It is
well substantiated that alkyl groups lower the energy of the double bond through
hyperconjugation. Saytzeff's rule is, however, not necessarily obeyed when the
carbocation remains part of an intimate ion pair. For example, elimination of
HX from
e7 See note 64, p. 355.
" (a) T. Cohen and A. R. Daniewski, J. Amer. Chem. Soc., 91, 533 (1969); (b) P. S. Skell and W. L.
Hall, J. Amer. Chem. Soc., 85, 2851 (1963).
69 See note 68(b).
70 For a similar example, see Humski, et al., J. Amer. Chem. Soc., 96, 6186 (1974).