Page 367 - Mechanism and Theory in Organic Chemistry
P. 367
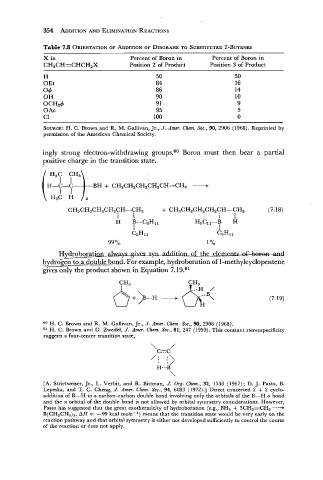
Table 7.8 ORIENTATION ADDITION OF DIBORANE SUBSTITUTED 2-BUTENES
OF
TO
X in Percent of Boron in Percent of Boron in
CH,CH=CHCH,X Position 2 of Product Position 3 of Product
H
OEt
o+
OH
OCH2+
0 Ac
C1
SOURCE: H. C. Brown and R. M. Gallivan, Jr., J. Amer. Chem. Soc., 90, 2906 (1968). Reprinted by
permission of the American Chemical Society.
ingly strong electron-withdrawing groups.60 Boron must then bear a partial
positive charge in the transition state.
. .
Hydroboration a l w ~ ~ e ~ l e ~ e u a d
hydrokFn to a double bond. For example, hydroboration of 1-methylcyclopentene
gives only the product shown in Equation 7. 19.61
H. C. Brown and R. M. Gallivan, Jr., J. Amer. Chem. Soc., 90, 2906 (1968).
61 H. C. Brown and G. Zweifel, J. Amer. Chem. Soc., 81, 247 (1959). This constant stereospecificity
suggests a four-center transition state,
[A. Strietweiser, Jr., L. Verbit, and R. Bittman, J. Org. Chem., 32, 1530 (1967); D. J. Pasto, B.
Lepeska, and T. C. Cheng, J. Amer. Chem. Soc., 94, 6083 (1972).] Direct concerted 2 + 2 cyclo-
addition of B-H to a carbon-carbon double bond involving only the orbitals of the B-H (I bond
and the TI orbital of the double bond is not allowed by orbital symmetry considerations. However,
Pasto has suggested that the great exothermicity of hydroboration (e.g., BH, + 3CH2=CH2 -+=
B(CH2CH,),, AH = -99 kcal mole-l) means that the transition state would be very early on the
reaction pathway and that orbital symmetry is either not developed sufficiently to control the course
of the reaction or does not apply.