Page 362 - Mechanism and Theory in Organic Chemistry
P. 362
Electrophilic Addition to Double and Triple Bonds 349
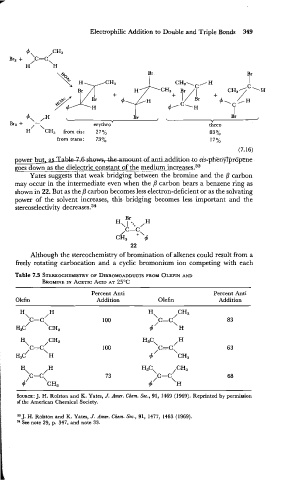
I Br
I
Bra + 4\-/H < Br j \ Y J
/-\ erythroY threo
H CH3 from cis: 27% 83 %
from trans: 73% 17%
(7.16)
power but. asT;lhlpmamt danthe addition addition0 cis-phenylpropime
goes down as the dielectric con$a-n~~of the mediumin~r_eases.~~
--
Yates suggests that weak bridging between the bromine and the fl carbon
may occur in the intermediate even when the fl carbon bears a benzene ring as
shown in 22. But as the P carbon becomes less electron-deficient or as the solvating
power of the solvent increases, this bridging becomes less important and the
stereoselectivity decreases.34
Although the stereochemistry of bromination of alkenes could result from a
freely rotating carbocation and a cyclic bromonium ion competing with each
OF
Table 7.5 STEREOCHEMISTRY DIBROMOADDUCTS PROM OLEFIN AND
BROMINE IN ACETIC ACID AT 25OC
Percent Anti Percent Anti
Addition Olefin Addition
SOURCE:
J. H. Rolston and K. Yates, J. Amer. Chem. Sac., 91, 1469 (1969). Reprinted by permission
of the American Chemical Society.
99 J. H. Rolston and K. Yates, J. Amer. Chem. Sac., 91, 1477, 1483 (1969).
See note 29, p. 347, and note 33.