Page 357 - Mechanism and Theory in Organic Chemistry
P. 357
cquation and anti-HOAc adducts, resp:ct_i_vely, are
. .
-. - -
termolecular processes with t r vl2adB.
.--
Such mechanisms are called Ad3 (addition-termolecular). Ad3 transition states
analogous to 12 and 13 but leading to svn adducts afeeim-by the stcriS
requirements of the addends.,, Thus increased chloride ion concentration in-
creases the contribution of the second term of the rate equation relative to the
other two, and anti-HC1 adduct is formed more rapidly thin syn-HC1 or -HOAc
adducts.
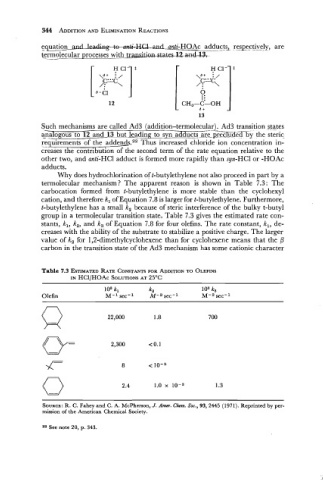
Why does hydrochlorination of t-butylethylene not also proceed in part by a
termolecular mechanism? The apparent reason is shown in Table 7.3: The
carbocation formed from t-butylethylene is more stable than the cyclohexyl
cation, and therefore k, of Equation 7.8 is larger for t-butylethylene. Furthermore,
t-butylethylene has a small k, because of steric interference of the bulky t-butyl
group in a termolecular transition state. Table 7.3 gives the estimated rate con-
stants, k,, k,, and k, of Equation 7.8 for four olefins. The rate constant, k,, de-
creases with the ability of the substrate to stabilize a positive charge. The larger
value of k, for 1,2-dimethylcyclohexene than for cyclohexene means that the P
carbon in the transition state of the Ad3 mechanism has some cationic character
Table 7.3 ESTIMATED F~TE CONSTANTS FOR ADDITION OLEFINS
TO
IN HCl/HOAc So~uno~s 25OC
AT
loe k, ka lo8 k,
Olefin M-l sec-l M-a sec-l M-a sec-l
0 2.4 1.0 x lo-, 1.3
SOURCE: R. C. Fahey and C. A. McPherson, J. Amer. Chem. SOG., 93,2445 (1971). Reprinted by per-
mission of the American Chemical Society.
aa See note 20, p. 343.