Page 355 - Mechanism and Theory in Organic Chemistry
P. 355
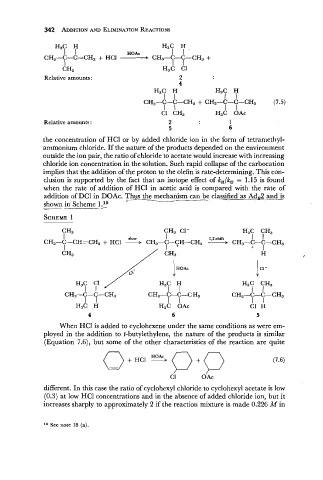
H3C H H3C H
I I HOAc I 1 '
CH3-C-C=CH2 + HCl CH3-C-C-CH3 +
I I I
CH3 H,C C1
Relative amounts: 2
4
H3C H H3C H
I I I I
CH3-C-C-CH3 + CH3-C-C-CH3 (7.5)
I I I I
C1 CH3 H3C OAc
Relative amounts : 2 1
5 6
the concentration of HC1 or by added chloride ion in the form of tetramethyl-
ammonium chloride. If the nature of the products depended on the environment
outside the ion pair, the ratio of chloride to acetate would increase with increasing
chloride ion concentration in the solution. Such rapid collapse of the carbocation
implies that the addition of the proton to the olefin is rate-determining. This con-
clusion is supported by the fact that an isotope effect of k,/k, = 1.15 is found
when the rate of addition of HC1 in acetic acid is compared with the rate of
addition of DC1 in DOAc. Thus - . the mechanism can -- be classified as Adp2 and is
shown in Scheme lLQ
CH3 CH, C1- H,C CH,
I slow I 1.2-shift 1 1
CH,-C-CH=CH, + HCl + CH,-C-CH-CH, ---+ CH,-C-C-CH,
I I + I
CH3 / CH3 + H
H,C C1 / H,C H H3C CH3
I I I I I I
CH3-C-(2-CH3 CH3-C-C-C H3 CH C-C-CH,
I I I I 3-~ I
H3C H H3C OAC C1 H
4 6 5
When HC1 is added to cyclohexene under the same conditions as were em-
ployed in the addition to t-butylethylene, the nature of the products is similar
(Equation 7.6), but some of the other characteristics of the reaction are quite
+ HCl +
different. In this case the ratio of cyclohexyl chloride to cyclohexyl acetate is low
(0.3) at low HC1 concentrations and in the absence of added chloride ion, but it
increases sharply to approximately 2 if the reaction mixture is made 0.226 M in
lo See note 18 (a).