Page 360 - Mechanism and Theory in Organic Chemistry
P. 360
Electrophilic Addition to Double and Triple Bonds 347
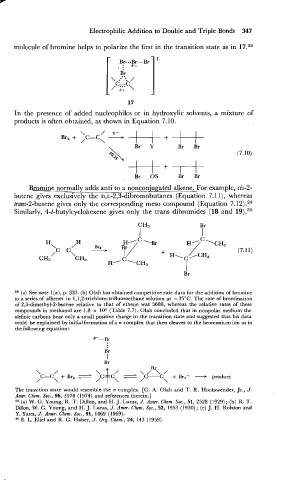
molecule of bromine helps to polarize the first in the transition state as in 17.28
In the presence of added nucleophiles or in hydroxylic solvents, a mixture of
products is often obtained, as shown in Equation 7.10.
Bromine normally adds anti - to a nonconiugated alkene. For example, cis-2-
butene gives exclusively the D,L-2,s-dibromobutanes (Equation 7.1 l), whereas
trans-2-butene gives only the corresponding meso compound (Equation 7.12) .29
Similarly, 4-t-butylcyclohexene gives only the trans dibromides (18 and 19).30
(a) See note I (a), p. 337. (b) Olah has obtained competitive rate data for the addition of bromine
to a series of alkenes in 1,1,2-trichloro-trifluoroethane solution at - 35'C. The rate of bromination
of 2,3-dimethyf-2-butene relative to that of ethene was 5680, whereas the relative rates of these
compounds in methanol are 1.8 x lo6 (Table 7.7). Olah concluded that in nonpolar medium the
olefinic carbons bear only a small positive charge in the transition state and suggested that his data
could be explained by initial formation of a n complex that then cleaves to the bromonium ion as in
the following equation :
Br
\ / -\/ \/
C=C + Br, = / ‘C+C< - /CC + Br, - - product
/ \ \
The transition state would resemble the n complex. [G. A. Olah and T. R. Hockswender, Jr., J.
Am. Chem. SOC., 96, 3574 (1974) and references therein.]
ag (a) W. G. Young, R. T. Dillon, and H. J. Lucas, J. Amer. Chem. Soc., 51, 2528 (1929); (b) R. T.
Dillon, W. G. Young, and H. J. Lucas, J. Amer. Chem. SOC., 52, 1953 (1930); (c) J. H. Rolston and
Y. Yates, J. Amer. Chem. SOC., 91, 1469 (1969).
E. L. Eliel and R. G. Haber, J. Org. Chem., 24, 143 (1959).