Page 363 - Mechanism and Theory in Organic Chemistry
P. 363
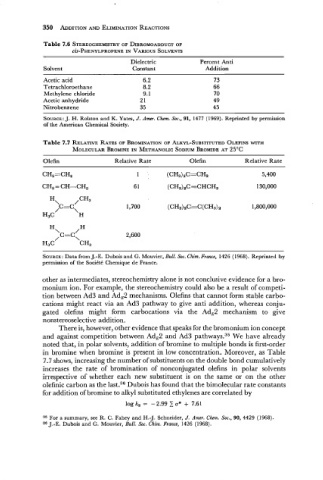
Table 7.6 STEREOCHEMISTRY DIBROMOADDUCT
OF
OF
C~S-PHENYLPROPENE VARIOUS SOLVENTS
IN
Dielectric Percent Anti
Solvent Constant Addition
Acetic acid 6.2 73
Tetrachloroethane 8.2 66
Methylene chloride 9.1 70
Acetic anhydride 2 1 49
Nitrobenzene 35 45
J.
SOURCE: H. Rolston and K. Yates, J. Amer. Chem. Soc., 91, 1477 (1969). Reprinted by permission
of the American Chemical Society.
Table 7.7 RELATIVE RATES OF BROMINATION OF ALKYL-SUBSTITUTED OLEFINS WITH
MOLECULAR BROMINE IN METHANOLIC SODIUM BROMIDE AT 25OC
Olefin Relative Rate Olefin Relative Rate
CH2 = CH-CH, 6 1 (CH3)aC=CHCH3 130,000
SOURCE: Data from J.-E. Dubois and G. Mouvier, Bull. Soc. Chim. France, 1426 (1968). Reprinted by
permission of the SociCtC Chemique de France.
other as intermediates, stereochemistry alone is not conclusive evidence for a bro-
monium ion. For example, the stereochemistry could also be a result of competi-
tion between Ad3 and AdE2 mechanisms. Olefins that cannot form stable carbo-
cations might react via an Ad3 pathway to give anti addition, whereas conju-
gated olefins might form carbocations via the AdE2 mechanism to give
nonstereoselective addition.
There is, however, other evidence that speaks for the bromonium ion concept
and against competition between AdE2 and Ad3 pathways.35 We have already
noted that, in polar solvents, addition of bromine to multiple bonds is first-order
in bromine when bromine is present in low concentration. Moreover, as Table
7.7 shows, increasing the number of substituents on the double bond cumulatively
increases the rate of bromination of nonconjugated olefins in polar solvents
irrespective of whether each new substituent is on the same or on the other
olefinic carbon as the last.36 Dubois has found that the bimolecular rate constants
for addition of bromine to alkyl substituted ethylenes are correlated by
35 For a summary, see R. C. Fahey and H.-J. Schneider, J. Amer. Chem. Soc., 90, 4429 (1968).
S6 J.-E. Dubois and G. Mouvier, Bull. Soc. Chim. France, 1426 (1968).