Page 368 - Mechanism and Theory in Organic Chemistry
P. 368
1,2-Elimination Reactions 355
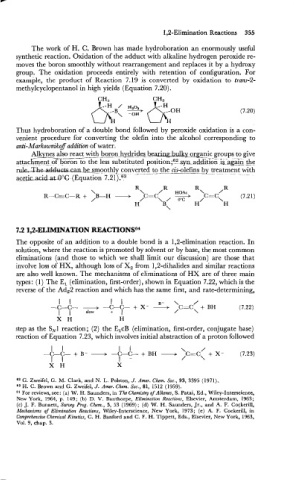
The work of H. C. Brown has made hydroboration an enormously useful
synthetic reaction. Oxidation of the adduct with alkaline hydrogen peroxide re-
moves the boron smoothly without rearrangement and replaces it by a hydroxy
group. The oxidation proceeds entirely with retention of configuration. For
example, the product of Reaction 7.19 is converted by oxidation to tram-2-
methylcyclopentanol in high yields (Equation 7.20).
Thus hydroboration of a double bond followed by peroxide oxidation is a con-
venient procedure for converting the olefin into the alcohol corresponding to
anti-Markownikoff addition of water.
with
Al-1so ___C___-- boron.h&id.exbearin~ bu lkv oznic ---- groups to give
react
attachment of boron to the less substituted p~sition;?~ synaddition icauain the
&Tke-Be_egmoothly with
-
aceticacid U C {Equation 7.2
The opposite of an addition to a double bond is a 1,2-elimination reaction. In
solution, where the reaction is promoted by solvent or by base, the most common
eliminations (and those to which we shall limit our discussion) are those that
involve loss of HX, although loss of X2 from 1,2-dihalides and similar reactions
are also well known. The mechanisms of eliminations of HX are of three main
types: (1) The El (elimination, first-order), shown in Equation 7.22, which is the
reverse of the Ad,2 reaction and which has the same first, and rate-determining,
I I 1 1 B- \ /
-C-C- + -C-C- + X- + C=C + BH (7.22)
I 1 slow + I / \
X H H
step as the S,1 reaction; (2) the ElcB (elimination, first-order, conjugate base)
reaction of Equation 7.23, which involves initial abstraction of a proton followed
6a G. Zweifel, G. M. Clark, and N. L. Polston, J. Amer. Chem. Soc., 93, 3395 (1971).
63 H. C. Brown and G. Zweifel, J. Amer. Chem. Soc., 81, 1512 (1959).
" For reviews, see: (a) W. H. Saunders, in The Chemistry of Alkenes, S. Patai, Ed., Wiley-Interscience,
New York, 1964, p. 149; (b) D. V. Banthorpe, Elimination Reactions, Elsevier, Amsterdam, 1963;
(c) J. F. Bunnett, Survey Prog. Chem., 5, 53 (1969); (d) W. H. Saunders, Jr., and A. F. Cockerill,
Mechanisms of Elimination Reactions, Wiley-Interscience, New York, 1973; (e) A. F. Cockerill, in
Comfirehensive Chemical Kinetics, C. H. Banford and C. F. H. Tippett, Eds., Elsevier, New York, 1963,
Vol. 9, chap. 3.