Page 369 - Mechanism and Theory in Organic Chemistry
P. 369
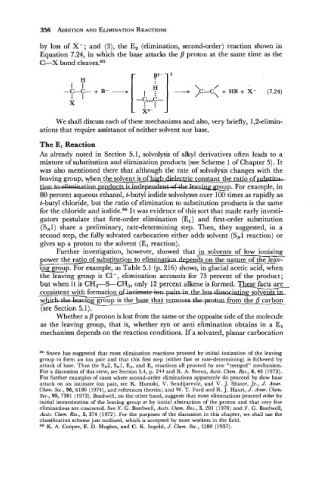
by loss of X-; and (3), the E, (elimination, second-order) reaction shown in
Equation 7.24, in which the base attacks the /3 proton at the same time as the
C--X bond cleaves.65
We shall discuss each of these mechanisms and also, very briefly, 1,2-elimin-
ations that require assistance of neither solvent nor base.
The El Reaction
As already noted in Section 5.1, solvolysis of alkyl derivatives often leads to a
mixture of substitution and elimination products (see Scheme 1 of Chapter 5). It
was also mentioned there that although the rate of solvolysis changes with the
leaving group, when-the solvent is of hi&ddatric constant the ratio of &
.. .
-0ducts is -up. For example, in
80 percent aqueous ethanol, t-butyl iodide solvolyzes over 100 times as rapidly as
t-butyl chloride, but the ratio of elimination to substitution products is the same
for the chloride and iodide.66 It was evidence of this sort that made early investi-
gators postulate that first-order elimination (El) and first-order substitution
(S,l) share a preliminary, rate-determining step. Then, they suggested, in a
second step, the fully solvated carbocation either adds solvent (S,1 reaction) or
gives up a proton to the solvent (El reaction).
Further investigation, however, showed that &_solvents of low ionizing
power the ratio of substitution to eliminatiandepends on the nature of thejcav-'
'Gig; group. For example, as Table 5.1 (p. 2 16) shows, in glacial acetic acid, when
the leaving group is C1-, elimination accounts for 73 percent of the product;
only 12 percent alkene is formed. T-facts
but when it is CH3-S-CH,, . . . . . .. are
consistent with f o r m a t l o n g solvrl..nrlannq-
w the * group is the base that r-from - the B carbon-
ion-
gect
Whether a /3 proton is lost from the same or the opposite side of the molecule
as the leaving group, that is, whether syn or anti elimination obtains in a El
mechanism depends on the reaction conditions. If a solvated, planar carbocation
85 Sneen has suggested that most elimination reactions proceed by initial ionization of the leaving
group to form an ion pair and that this first step (either fast or rate-determining) is followed by
attack of base. Thus the SN2, S,1, E2, and E, reactions all proceed by one "merged" mechanism.
For a discussion of this view, see Section 5.4, p. 244 and R. A. Sneen, Accls. Chem. Res., 6, 46 (1973).
For further examples of cases where second-order eliminations apparently do proceed by slow base
attack on an intimate ion pair, see K. Humski, V. SendijareviC, and V. J. Shiner, Jr., J. Amer.
Chem. Sac., 96, 6186 (1974), and references therein; and W. T. Ford and R. J. Hauri, J. Amer. Chem.
Sac., 95, 7381 (1973). Bordwell, on the other hand, suggests that most eliminations proceed either by
initial isomerization of the leaving group or by initial abstraction of the proton and that very few
eliminations are concerted. See F. G. Bordwell, Accls. Chem. Res., 3, 281 (1970) and F. G. Bordwell,
Accls. Chem. Res., 5, 374 (1972). For the purposes of the discussion in this chapter, we shall use the
classification scheme just outlined, which is accepted by most workers in the field.
88 K. A. Cooper, E. D. Hughes, and C. K. Ingold, J. Chem. Sac., 1280 (1937).