Page 364 - Mechanism and Theory in Organic Chemistry
P. 364
Electrophilic Addition to .Double and Triple Bonds 351
where 1 o* represents the sum of the Taft o* values for the four substituents
on the double bond.37 Thus, in the transition state the positive charge is distrib-
uted over both carbons of the double bond-a very different situation from
that obtaining in hydration or hydrochlorination of double bonds (see, for
example, Table 7.1).
From the stereochemical evidence we might expect that the effect of sub-
stituents on the rate of bromination of substituted styrenes in polar solvents would
not be c~mulative.~~ And, indeed, 23, 24, and 25, when brominated under the
conditions of Table 7.7, have the relative rates shown. Furthermore, the logs of
the rates of bromination of ring-substituted styrenes show a linear correlation
4CH=CH, 4CH3C=CH2 4CH=CHCH3
23 24 25
Relative rates: 1 87 25
with the o+ constants of the substituents with slope p z - 4.5.39
Another piece of evidence for the bromonium ion is that addition is less
regiospecific when bromine is the electrophile then when H30+ attacks. With
molecular bromine we cannot, of course, observe the site at which the original
electrophilic bromine attacks, but with unsymmetricalreactants such as HOBr or
BrCl we can. Thus, for example, the addition of BrCl to propene in aqueous HC1
gives only 54 percent of the Markownikoff addition product (26) and 46 percent
of the anti-h4arkownikoff product (27).40 The chloride ion apparently has the
CH3-CH-CH2 CH3-CH-CHz
I I I I
C1 Br Br C1
26 27
choice of attacking either of two carbons, both of which carry approximately equal
positive charges-a situation that would exist in the bromonium ion.
Recently Olah has observed the unsubstituted bromonium ion and several
alkylated bromonium ions by nmr spectroscopy after dissolving or-bromohalides
in SbF,-SO2 solution at low temperature^.^^ All four hydrogens of the unsub-
stituted ion were equivalent.
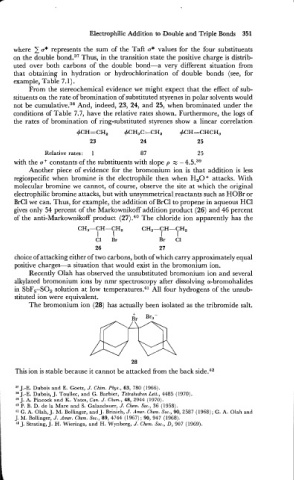
The bromonium ion (28) has actually been isolated as the tribromide salt.
28
This ion is stable because it cannot be attacked from the back side.42
37 J.-E. Dubois and E. Goetz, J. Chim. Phys., 63, 780 (1966).
3B J.-E. Dubois, J. Toullec, and G. Barbier, Tetrahedron Lett., 4485 (1970).
39 J. A. Pincock and K. Yates, Can. J. Chem., 48, 2944 (1970).
40 P. B. D. de la Mare and S. Galandauer, J. Chem. Soc., 36 (1958).
41 G. A. Olah, J. M. Bollinger, and J. Brinich, J. Amer. Chem. Soc., 90, 2587 (1968); G. A. Olah and
J. M. Bollinger, J. Amer. Chem. Soc., 89, 4744 (1967); 90, 947 (1968).
4a J. Strating, J. H. Wieringa, and H. Wynberg, J. Chem. Soc., D, 907 (1969).