Page 359 - Mechanism and Theory in Organic Chemistry
P. 359
-
\
4 /\/\// +HBr
-
3
RX
ROAc
-
2
r
-
1
.+:-8.--0-0.-• . .-
+
HCI
0 ,
I l l I I I I I I I
OO 0.5 1D
IHXI
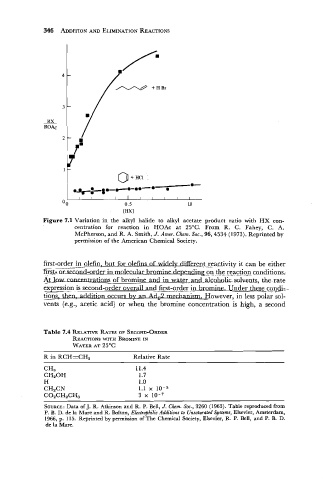
Figure 7.1 Variation in the alkyl halide to alkyl acetate product ratio with HX con-
centration for reaction in HOAc at 25OC. From R. C. Fahey, C. A.
McPherson, and R. A. Smith, J. Amer. Chem. Soc., 96,4534 (1973). Reprinted by
permission of the American Chemical Society.
e
first-order in olefin, but for olefins of w ~ y ~ reactivity t
~ it can be either
fir& or-d-order in molecular bro-i_n~on the reactio-n-conditions.
A-ations of bromine and in ~ater__a~adalcaholic solvents, the rate
expression is second-order overall and first-orderinbromiw. Under these con&: -
tions, then.tion occurs by an Arlu7mecha.nism. However, in less polar sol-
vents (e.g., acetic acid) or when the bromine concentration is high, a second
Table 7.4 RELATIVE RATES OF SECOND-ORDER
REACTIONS
WITH BROMINE IN
WATER AT 25OC
R in RCH=CH, Relative Rate
CH3 11.4
CHzOH 1.7
H 1 .o
CHzCN 1.1 x lo-3
CO2CH2CH3 3 x lo-'
SOURCE: Data of J. R. Atkinson and R. P. Bell, J. Chem. Soc., 3260 (1963). Table reproduced from
P. B. D. de la Mare and R. Bolton, Electrophilic Additions to Unsaturated System, Elsevier, Amsterdam,
1966, p. 115. Reprinted by permission of The Chemical Society, Elsevier, R. P. Bell, and P. B. D.
de la Mare.