Page 356 - Mechanism and Theory in Organic Chemistry
P. 356
Electrophilic Addition to Double and Triple Bonds 343
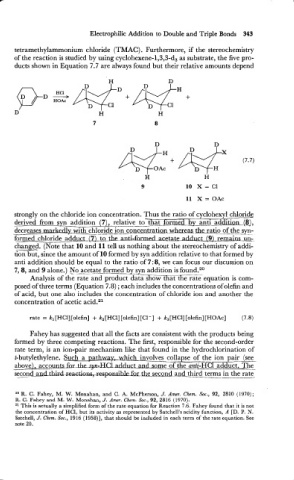
tetramethylammonium chloride (TMAC). Furthermore, if the stereochemistry
of the reaction is studied by using cyclohexene-1,3,3-d, as substrate, the five pro-
ducts shown in Equation 7.7 are always found but their relative amounts depend
HOAc
D H H
7 8
11 X = OAc
strongly on the chloride ion concentration. Thus the ratio of cyclohexyl chloride
derived from syn addition (71, relative to-<
decreases d y with chloride ion con~entrati~~hm ratio of the sysyn-
the
-----
11
ride adduct (Lt_o-.~L~anti-fnrmed acetate &ct (9) remaiwn-
chxd. (Note that 10 and 11 tell us nothing about the stereochemistry of addi-
6on but. since the amount of 10 formed by svn addition relative to that formed by
, ,
anti addition should be equal to the ratio of 7 : 8, we can focus our discussion on
7,8, and 9 alone.) No acetate formed by syn addition is found.20
- --
Analysis of the rate and product data &o~t5~~eequation
is
com-
posed of three terms (Equation 7.8) ; each includes the concentrations of olefin and
of acid, but one also includes the concentration of chloride ion and another the
concentration of acetic acid.21
rate = k, [HCl] [olefin] + k,[HCl] [olefin] [Cl-] + k3[HC1] [olefin] [HOAc] (7.8)
Fahey has suggested that all the facts are consistent with the products being
formed by three competing reactions. The first, responsible for the second-order
rate term, is an ion-pair mechanism like that found in the hydrochlorination of
t-butylethylene. Such a pathway, which involves collapse of the ion pair (see
above), accountsh._theqtz--t and some of the anti-HC1 adduct. a he-
second
and
sec_~,~n~a~d~-~ns,~far~hr: in the rag
terms
third
ao R. C. Fahey, M. W. Monahan, and C. A. McPherson, J. Amer. Chem. Soc., 92, 2810 (1970);
R. C. Fahey and M. W. Monahan, J. Amer. Chem. SOC., 92, 2816 (1970).
This is actually a simplified form of the rate equation for Reaction 7.6. Fahey found that it is not
the concentration of HCl, but its activity as represented by Satchell's acidity function, A [D. P. N.
Satchell, J. Chem. SOC., 1916 (1958)], that should be included in each term of the rate equation. See
note 20.