Page 361 - Mechanism and Theory in Organic Chemistry
P. 361
(7.13)
Br H
18 19
major minor
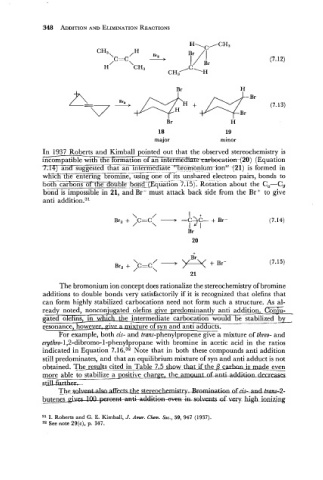
In 1937 Roberts and Kimball pointed out that the observed stereochemistry is
incom~atible with the formation of an intermeciiatemhatki201 (Equation
,,
,
I. 14) and suggested &at an intermediiite-Tr6inonium- ior? f 21) is formed in
-
-
whTCE-die entering bromine, using one of-& unshared electron pairs, bonds to
~
b
e
l
boxcarbons ot ~ ~ ~ b0ri~@~uat;bn7.15)~ Rotation about the C,-C,
bond is impossible in 21, and Br- must attack back side from the Br+ to give
anti addition.31
The bromonium ion concept does rationalize the stereochemistry of bromine
additions to double bonds very satisfactorily if it is recognized that olefins that
can form highly stabilized carbocations need not form such a structure. As al-
ready noted, nbnconjugated olefins give predominantly anti addition. s-
gated olefins, in which the intermediate carbocation w o n stabilized by
---- -
yesonance, however, give a mixture of syn and anti adducts.
For example, both cis- and trans-phenylpropene gives mixture of threo- and
eythro-l,2-dibromo-l-phenylpropane with bromine in acetic acid in the ratios
indicated in Equation 7.16.32 Note that in both these compounds anti addition
still predominates, and that an equilibrium mixture of syn and anti adduct is not
obtained. The results cited in Table 7.5sh~w.tkartifthe chis made even
. .
more able to s t a b i l i t - a e s
st-
The salYentalsaaffectsthe stereoch~+W&_af~is-a&m-2-
butenes s i v & e w ~U-SO~V~XI~S very hqh ionizing
of
I. Roberts and G. E. Kimball, J. Amcr. Chcm. SOG., 59, 947 (1937).
32 See note 29(c), p. 347.