Page 379 - Mechanism and Theory in Organic Chemistry
P. 379
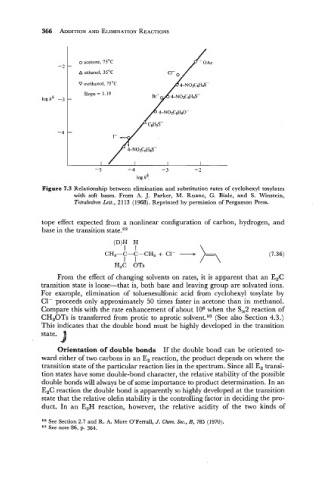
- o acetone, 75'C
A ethanol, 3S°C
V methanol, 7S°C
Slope = 1.19
log kE -
-
I
- 5 -4 - 3 -
7
log k?
Figure 7.3 Relationship between elimination and substitution rates of cyclohexyl tosylates
with soft bases. From A. J. Parker, M. Ruane, G. Biale, and S. Winstein,
Tetrahedron Lett., 21 13 (1968). Reprinted by permission of Pergamon Press.
tope effect expected from a nonlinear configuration of carbon, hydrogen, and
base in the transition state.92
CH,-C-C-CH, + CI- + (7.36)
I I
H,C OTs
From the effect of changing solvents on rates, it is apparent that an E2C
transition state is loose-that is, both base and leaving group are solvated ions.
For example, elimination of toluenesulfonic acid from cyclohexyl tosylate by
C1- proceeds only approximately 50 times faster in acetone than in methanol.
Compare this with the rate enhancement of about lo6 when the S,2 reaction of
CH,OTs is transferred from protic to aprotic solvent.93 (See also Section 4.3.)
This indicates that the double bond must be highly developed in the transition
J
Orientation of double bonds If the double bond can be oriented to-
ward either of two carbons in an E2 reaction, the product depends on where the
transition state of the particular reaction lies in the spectrum. Since all E2 transi-
tion states have some double-bond character, the relative stability of the possible
double bonds will always be of some importance to product determination. In an
E2C reaction the double bond is apparently so highly developed at the transition
state that the relative olefin stability is the controlling factor in deciding the pro-
duct. In an E2H reaction, however, the relative acidity of the two kinds of
Oa See Section 2.7 and R. A. More O'Ferrall, J. Chem. Soc., B, 785 (1970).
O3 See note 86, p. 364.