Page 383 - Mechanism and Theory in Organic Chemistry
P. 383
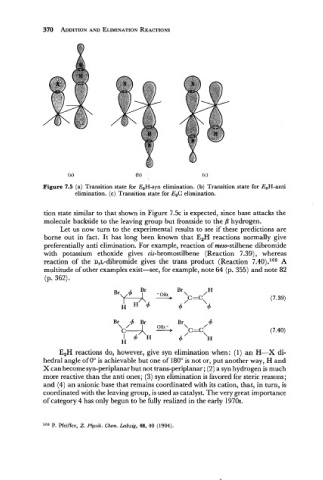
Figure 7.5 (a) Transition state for E2H-syn elimination. (b) Transition state for E2H-anti
elimination. (c) Transition state for E,C elimination.
tion state similar to that shown in Figure 7.5~ expected, since base attacks the
is
molecule backside to the leaving group but frontside to the /3 hydrogen.
Let us now turn to the experimental results to see if these predictions are
borne out in fact. It has long been known that E2H reactions normally give
preferentially anti elimination. For example, reaction of meso-stilbene dibromide
with potassium ethoxide gives cis-bromostilbene (Reaction 7.39), whereas
reaction of the D,L-dibromide gives the trans product (Reaction 7.40).lo2 A
multitude of other examples exist-see, for example, note 64 (p. 355) and note 82
(p. 362).
E2H reactions do, however, give syn elimination when: (1) an H-X di-
hedral angle of 0" is achievable but one of 180" is not or, put another way, H and
X can become syn-periplanar but not trans-periplanar ; (2) a syn hydrogen is much
more reactive than the anti ones; (3) syn elimination is favored for steric reasons;
and (4) an anionic base that remains coordinated with its cation, that, in turn, is
coordinated with the leaving group, is used as catalyst. The very great importance
of category 4 has only begun to be fully realized in the early 1970s.
lo2 P. Pfeiffer, 2. Physik. Chem. Leibrig, 48, 40 (1904).