Page 388 - Mechanism and Theory in Organic Chemistry
P. 388
1,2-Elimination Reactions 375
- 5 -4 - -2
3
log kJ
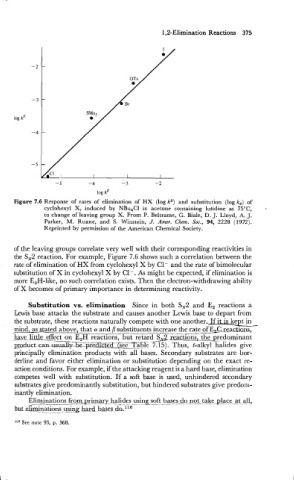
Figure 7.6 Response of rates of elimination of HX (log kE) and substitution (log k,) of
cyclohexyl X, induced by NBu,CI in acetone containing lutidine at 75OC, -
to change of leaving group X. From P. Beltrame, G. Biale, D. J. Lloyd, A. J.
Parker, M. Ruane, and S. Winstein, J. Amer. Chem. Soc., 94, 2228 (1972).
Reprinted by permission of the American Chemical Society.
of the leaving groups correlate very well with their corresponding reactivities in
the S,2 reaction. For example, Figure 7.6 shows such a correlation between the
rate of elimination of HX from cyclohexyl X by C1- and the rate of bimolecular
i
substitution of X in cyclohexyl X by C1-. As might be expected, if elimination is
more E,H-like, no such correlation exists. Then the electron-withdrawing ability
of X becomes of primary importance in determining reactivity.
Substitution vs. elimination Since in both S,2 and E, reactions a
Lewis base attacks the substrate and causes another Lewis base to depart from
the substrate, these reactions naturally compete with one another. If it is kept in
mind, as stated above, that a and /3 substituents increase the rate of&~-reacc
-----
-- ----
--
Lave lifrte effect on E,H reactions, but retard SS2 reactions,_ttejredominant
--
--
*t- 'ted-KeETii'EiIe 7.15). Thus, t-alkyl halides give
principally elimination products with all bases. Secondary substrates are bor-
derline and favor either elimination or substitution depending on the exact re-
action conditions. For example, if the attacking reagent is a hard base, elimination
competes well with substitution. If a soft base is used, unhindered secondary
substrates give predominantly substitution, but hindered substrates give predom-
inantly elimination.
Eliminations from primnry halides __-. using soft ______-__ bases do not takxlace at all,
but -@=;hard baser do.lL6
"' See note 95, p. 368.