Page 386 - Mechanism and Theory in Organic Chemistry
P. 386
1,2-Elimination Reactions 373
49c would cause the two alkyl groups to be eclipsed in the transition state. These
are the two pathways for syn elimination leading to cis olefin. Syn elimination
from 49a and loss of H, from 49c allows the two alkyl groups to be anti in
the transition state. Therefore syn elimination gives trans olefin.lo7 In accord with
Saunders' theory, other bulky ammonium salts also show the syn-anti dichotomy,
whereas unhindered ones appear to eliminate entirely anti.lo8
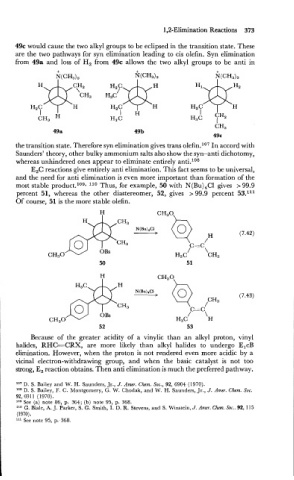
E,C reactions give entirely anti elimination. This fact seems to be universal,
and the need for anti elimination is ,even more important than formation of the
most stable product.log. 11° Thus, for example, 50 with N(Bu),Cl gives > 99.9
percent 51, whereas the other diastereomer, 52, gives > 99.9 percent 53.ll1
Of course, 51 is the more stable olefin.
Because of the greater acidity of a vinylic than an alkyl proton, vinyl
halides, RHC=CRX, are more likely than alkyl halides to undergo E,cB
elimination. However, when the proton is not rendered even more acidic by a
vicinal electron-withdrawing group, and when the basic catalyst is not too
strong, E, reaction obtains. Then anti elimination is much the preferred pathway.
loT D. S. Bailey and W. H. Saunders, Jr., J. Amer. Chem. Soc., 92, 6904 (1970).
lo8 D. S. Bailey, F. C. Montgomery, G. W. Chodak, and W. H. Saunders, Jr., J. Amer. Chem. Soc.
92, 6911 (1970).
log See (a) note 86, p. 364; (b) note 95, p. 368.
' "O G. Biale, A. J. Parker, S. G. Smith, I. D. R. Stevens, and S. Winstein, J. Amer. Chem. Soc.. 92, 115
(1970).
ll1 See note 95, p. 368.