Page 385 - Mechanism and Theory in Organic Chemistry
P. 385
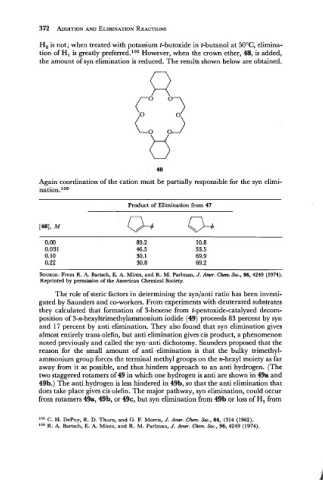
H, is not; when treated with potassium t-butoxide in t-butanol at 50°C, elimina-
tion of HI is greatly preferred.lo5 However, when the crown ether, 48, is added,
the amount of syn elimination is reduced. The results shown below are obtained.
Again coordination of the cation must be partially responsible for the syn elimi-
nation.lo8
Product of Elimination from 47
SOURCE: From R. A. Bartsch, E. A. Mintz, and R. M. Parlrnan, J: Am. Ch. Soc., 96, 4249 (1974).
Reprinted by permission of the American Chemical Society.
The role of steric factors in determining the synlanti ratio has been investi-
gated by Saunders and co-workers. From experiments with deuterated substrates
they calculated that formation of 3-hexene from t-pentoxide-catalyzed decom-
position of 3-n-hexyltrimethylammonium iodide (49) proceeds 83 percent by syn
and 17 percent by anti elimination. They also found that syn elimination gives
almost entirely trans olefin, but anti elimination gives cis product, a phenomenon
noted previously and called the syn-anti dichotomy. Saunders proposed that the
reason for the small amount of anti elimination is that the bulky trimethyl-
ammonium group forces the terminal methyl groups on the n-hexyl moiety as far
away from it as possible, and thus hinders approach to an anti hydrogen. (The
two staggered rotamers of 49 in which one hydrogen is anti are shown in 49a and
49b.) The anti hydrogen is less hindered in 49b, so that the anti elimination that
does take place gives cis olefin. The major pathway, syn elimination, could occur
from rotamers 49a, 49b, or 49c, but syn elimination from 49b or loss of HI from
'OK C. H. DePuy, R. D. Thurn, and G. F. Morris, J. Amcr. Chern. Soc., 84, 1314 (1962).
lo6 R. A. Bartsch, E. A. Mintz, and R. M. Parlman, J. Arncr. Chcrn. Soc., 96, 4249 (1974).