Page 384 - Mechanism and Theory in Organic Chemistry
P. 384
1,2-Elimination Reactions 371
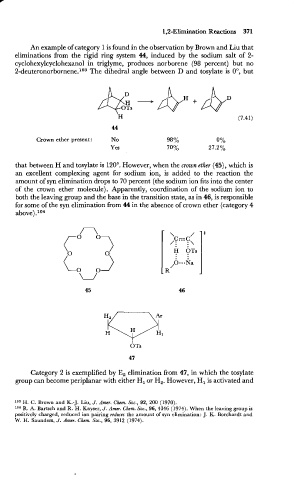
An example of category 1 is found in the observation by Brown and Liu that
eliminations from the rigid ring system 44, induced by the sodium salt of 2-
cyclohexylcyclohexanol in triglyme, produces norborene (98 percent) but no
The
2-de~teronorbornene.~~~ dihedral angle between D and tosylate is O", but
Crown ether present: No
Yes
that between H and tosylate is 120". However, when the crown ether (45), which is
an excellent complexing agent for sodium ion, is added to the reaction the
amount of syn elimination drops to 70 percent (the sodium ion fits into the center
of the crown ether molecule). Apparently, coordination of the sodium ion to
both the leaving group and the base in the transition state, as in 46, is responsible
for some of the syn elimination from 44 in the absence of crown ether (category 4
above) .Io4
OTs
Category 2 is exemplified by E, elimination from 47, in which the tosylate
group can become periplanar with either H, or H,. However, H, is activated and
lo3 H. C. Brown and K.-J. Liu, J. Amer. Chem. Sac., 92, 200 (1970).
lo4 R. A. Bartsch and R. H. Kayser, J. Amer. Chem. Sac., 96, 4346 (1974). When the leaving group is
positively charged, reduced ion pairing reduces the amount of syn elimination: J. K. Borchardt and
W. H. Saunders, J. Amr. Chem. Sac., 96, 3912 (1974).