Page 382 - Mechanism and Theory in Organic Chemistry
P. 382
1,2-Elimination Reactions 369
-
0.6
-0.8 -
-
-1.0
AAG~ (kcal mole-')
-1.2 -
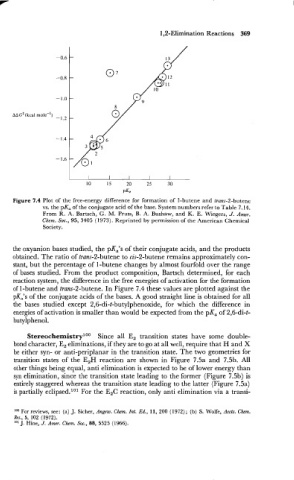
Figure 7.4 Plot of the free-energy difference for formation of I-butene and trans-2-butene
vs. the pK, of the conjugate acid of the base. System numbers refer to Table 7.14.
From R. A. Bartsch, G. M. Pruss, B. A. Bushaw, and K. E. Wiegers, J. Amer.
Chem. Soc., 95, 3405 (1973). Reprinted by permission of the American Chemical
Society.
the oxyanion bases studied, the pKa's of their conjugate acids, and the products
obtained. The ratio of trans-2-butene to cis-2-butene remains approximately con-
stant, but the percentage of 1 -butene changes by almost fourfold over the range
of bases studied. From the product composition, Bartsch determined, for each
reaction system, the difference in the free energies of activation for the formation
of 1-butene and tram-2-butene. In Figure 7.4 these values are plotted against the
pK,'s of the conjugate acids of the bases. A good straight line is obtained for all
the bases studied except 2,6-di-t-butylphenoxide, for which the difference in
energies of activation is smaller than would be expected from the pKa of 2,6-di-t-
butylphenol.
StereochemistryloO Since all E, transition states have some double-
bond character, E, eliminations, if they are to go at all well, require that H and X
be either syn- or anti-periplanar in the transition state. The two geometries for
transition states of the E,H reaction are shown in Figure 7.5a and 7.5b. All
other things being equal, anti elimination is expected to be of lower energy than
syn elimination, since the transition state leading to the former (Figure 7.5b) is
entirely staggered whereas the transition state leading to the latter (Figure 7.5a)
is partially eclipsed.lO' For the E,C reaction, only anti elimination via a transi-
loo For reviews, see: (a) J. Sicher, Angew. Chem. Int. Ed., 11, 200 (1972); (b) S. Wolfe, Accts. Chem.
Res., 5, 102 (1972).
lo' J. Hine, J. Amer. Chem. Sod., 88, 5525 (1966).