Page 387 - Mechanism and Theory in Organic Chemistry
P. 387
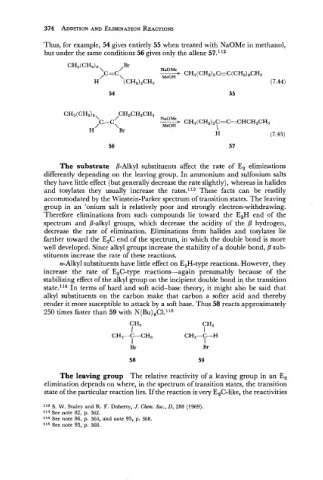
Thus, for example, 54 gives entirely 55 when treated with NaOMe in methanol,
but under the same conditions 56 gives only the allene 57.112
CHdCHz)z\ ,,Br NaOMe
/C=C A CH3(CH2), CGC(CH~)~CH~
\ MeOH
H (CH2)2CH3 (7.44)
CH3(CH2)2\ / CH2CH2CH3
NaOMe
/C ==C CH3(CH2)2C=C=CHCH2CH3
\ MeOH I
H Br
H (7.45)
56 57
The substrate /3-Alkyl substituents affect the rate of E2 eliminations
differently depending on the leaving group. In ammonium and sulfonium salts
they have little effect (but generally decrease the rate slightly), whereas in halides
and tosylates they usually increase the rates.l13 These facts can be readily
accommodated by the Winstein-Parker spectrum of transition states. The leaving
group in an 'onium salt is relatively poor and strongly electron-withdrawing.
Therefore eliminations from such compounds lie toward the E2H end of the
spectrum and /3-alkyl groups, which decrease the acidity of the /3 hydrogen,
decrease the rate of elimination. Eliminations from halides and tosylates lie
farther toward the E,C end of the spectrum, in which the double bond is more
well developed. Since alkyl groups increase the stability of a double bond, sub-
stituents increase the rate of these reactions.
a-Alkyl substituents have little effect on E,H-type reactions. However, they
increase the rate of E2C-type reactions-again presumably because of the
stabilizing effect of the alkyl group on the incipient double bond in the transition
state.l14 In terms of hard and soft acid-base theory, it might also be said that
alkyl substituents on the carbon make that carbon a softer acid and thereby
render it more susceptible to attack by a soft base. Thus 58 reacts approximately
250 times faster than 59 with N(Bu),C1.115
CH3 CH3
I I
CH3-C-CH3 CH3-C-H
I I
The leaving group The relative reactivity of a leaving group in an E2
elimination depends on where, in the spectrum of transition states, the transition
state of the particular reaction lies. If the reaction is very E2C-like, the reactivities
112 S. W. Staley and R. F. Doherty, J. Chem. SOG., D, 288 (1969).
113 See note 82, p. 362.
114 See note 86, p. 364, and note 95, p. 368.
116 See note 95, p. 368.