Page 69 - Mechanism and Theory in Organic Chemistry
P. 69
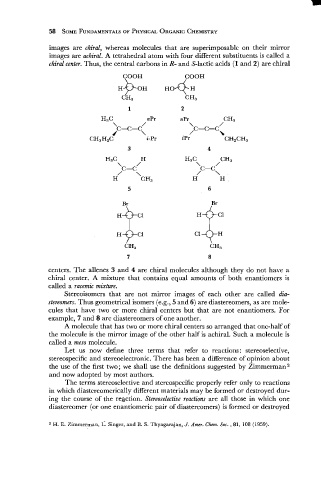
images are chiral, whereas molecules that are superimposable on their mirror
images are achiral. A tetrahedral atom with four different substituents is called a
chiral center. Thus, the central carbons in R- and S-lactic acids (1 and 2) are chiral
centers. The allenes 3 and 4 are chiral molecules although they do not have a
chiral center. A mixture that contains equal amounts of both enantiomers is
called a racemic mixture.
Stereoisomers that are not mirror images of each other are called dia-
stereomers. Thus geometrical isomers (e.g., 5 and 6) are diastereomers, as are mole-
cules that have two or more chiral centers but that are not enantiomers. For
example, 7 and 8 are diastereomers of one another.
A molecule that has two or more chiral centers so arranged that one-half of
the molecule is the mirror image of the other half is achiral. Such a molecule is
called a meso molecule.
Let us now define three terms that refer to reactions: stereoselective,
stereospecific and stereoelectronic. There has been a difference of opinion about
the use of the first two; we shall use the definitions suggested by Zimmerman2
and now adopted by most authors.
The terms stereoselective and stereospecific properly refer only to reactions
in which diastereomerically different materials may be formed or destroyed dur-
ing the course of the regction. Stereoselective reactions are all those in which one
diastereomer (or one enantiomeric pair of diastereomers) is formed or destroyed
H. E. Zimmerman, i. Singer, and B. S. Thyagarajan, J. Amer. Chem. Soc. ,81, 108 (1959).