Page 74 - Mechanism and Theory in Organic Chemistry
P. 74
Linear Free-Energy Relationships 63
log -
K
KO
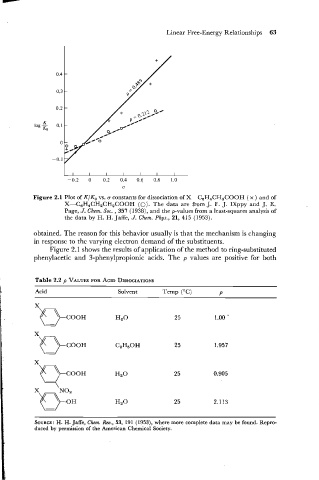
Figure 2.1 Plot of K/Ko vs. a constants for dissociation of X-C,H4CH2COOH ( x ) and of
X-C6H4CH,CH2COOH (0). data are from J. F. J. Dippy and J. E.
The
,
Page, J. Chem. SOG. 357 (19381, and the p-values from a least-squares analysis of
the data by H. H. Jaffe, J. Chem. Phys., 21, 415 (1953).
obtained. The reason for this behavior usually is that the mechanism is changing
in response to the varying electron demand of the substituents.
Figure 2.1 shows the results of application of the method to ring-substituted
phenylacetic and 3-phenylpropionic acids. The p values are positive for both
Table 2.2 p VALUES FOR ACID DISSOCIATIONS
Acid Solvent Temp ("C) p
SOURCE: H. H. Jaffe, Chem. Rev., 53, 191 (1953), where more complete data may be found. Repro-
duced by permission of the American Chemical Society.