Page 75 - Mechanism and Theory in Organic Chemistry
P. 75
64 SOME FUNDAMENTALS OF PHYSICAL ORGANIC CHEMISTRY
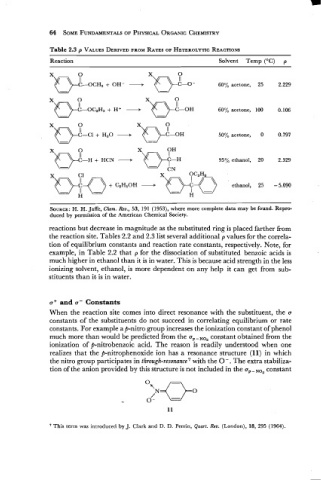
Table 2.3 p VALUES DERIVED FROM RATES OF HETEROLYTIC REACTIONS
Reaction Solvent Temp (OC) p
xQLI-oCH3 + "DL,- 60% acetone, 25 2.229
+
0.-
60% acetone, 100 0.106
- + H.0 + 50% acetone, 0 0.797
- + HCN + CN 95% ethanol, 20 2.329
"~p'o ethanol, 25 -5.090
+
C2H60H
H
SOURCE: H. H. Jaffe, Chem. Rev., 53, 191 (1953), where more complete data may be found. Repro-
duced by permission of the American Chemical Society.
reactions but decrease in magnitude as the substituted ring is placed farther from
the reaction site. Tables 2.2 and 2.3 list several additional p values for the correla-
tion of equilibrium constants and reaction rate constants, respectively. Note, for
example, in Table 2.2 that p for the dissociation of substituted benzoic acids is
much higher in ethanol than it is in water. This is because acid strength in the less
ionizing solvent, ethanol, is more dependent on any help it can get from sub-
stituents than it. is in water.
u+ and u- Constants
When the reaction site comes into direct resonance with the substituent, the u
constants of the substituents do not succeed in correlating equilibrium or rate
constants. For example ap-nitro group increases the ionization constant of phenol
much more than would be predicted from the constant obtained from the
ionization of p-nitrobenzoic acid. The reason is readily understood when one
realizes that the p-nitrophenoxide ion has a resonance structure (11) in which
the nitro group participates in through-resonance7 with the 0-. The extra stabiliza-
tion of the anion provided by this structure is not included in the up- constant
' This term was introduced by J. Clark and D. D. Perrin, Quart. Rev. (London), 18, 295 (1964).