Page 71 - Mechanism and Theory in Organic Chemistry
P. 71
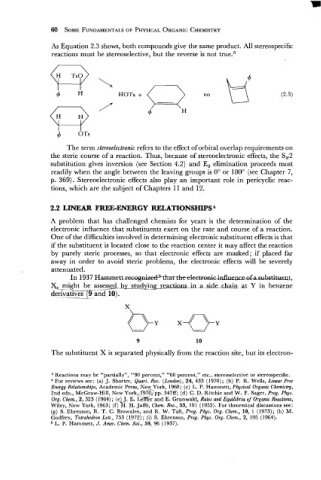
As Equation 2.3 shows, both compounds give the same product. All stereospecific
reactions must be stereoselective, but the reverse is not true.3
0
4
OTs
The term stereoelectronic refers to the effect of orbital overlap requirements on
the steric course of a reaction. Thus, because of stereoelectronic effects, the S,2
substitution gives inversion (see Section 4.2) and E, elimination proceeds most
readily when the angle between the leaving groups is 0" or 180" (see Chapter 7,
p. 369). Stereoelectronic effects also play an important role in pericyclic reac-
tions, which are the subject of Chapters 11 and 12.
2.2 LINEAR FREE-ENERGY RELATIONSHIPS
A problem that has challenged chemists for years is the determination of the
electronic influence that substituents exert on the rate and course of a reaction.
One of the difficulties involved in determining electronic substituent effects is that
if the substituent is located close to the reaction center it may affect the reaction
by purely steric processes, so that electronic effects are masked; if placed far
away in order to avoid steric problems, the electronic effects will be severely
attenuated. . .
In 1937 Hammett-e t,
thew-
X,-m@ht be as~sed bv s&u&ing redm ina sidechain at Y in benzene
derivatiVeS('§and lo).
-
The substituent X is separated physically from the reaction site, but its electron-
Reactions may be "partially", "90 percent," "60 percent," etc., stereoselective or stereospecific.
* For reviews see: (a) J. Shorter, Quart. Rev. (London), 24, 433 (1970); (b) P. R. Wells, Linear Free
Energy Relationships, Academic Press, New York, 1968; (c) L. P. Hammett, Physical Organic Chemistry,
2nd edn., McGraw-Hill, New ~ork,,l'97G~~. 347ff; (d) C. D. Ritchie and W. F. Sager, Prog. Phys.
Org. Chem., 2, 323 (1964); (el J. E. ~ker and E. Grunwald, Rates and Equilibria of Organic Reaclions,
Wiley, New York, 1963; (f) H. H. Jaff6, Chem. Rev., 53, 191 (1953). For theoretical discussions see:
(g) S. Ehrenson, R. T. C. Brownlee, and R. W. Taft, Prog. Phys. Org. Chem., 10, 1 (1973); (h) M.
Godfrey, Telrahedron Lett., 753 (1972); (i) S. Ehrenson, Prog. Phys. Org. Chem., 2, 195 (1964).
L. P. Harnmett, J. Amer. Chem. So;., 59, 96 (1937).