Page 70 - Mechanism and Theory in Organic Chemistry
P. 70
Stereochemistry 59
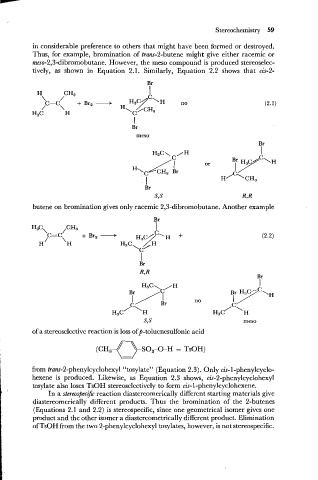
in considerable preference to others that might have been formed or destroyed.
Thus, for example, bromination of trans-2-butene might give either racemic or
meso-2,3-dibromobutane. However, the meso compound is produced stereoselec-
tively, as shown in Equation 2.1. Similarly, Equation 2.2 shows that cis-2-
butene on bromination gives only racemic 2,3-dibromobutane. Another example
of a stereoselective reaction is loss ofp-toluenesulfonic acid
(CH~OSO,-O-H = TsOH)
-
from trans-2-phenylcyclohexyl "tosylate" (Equation 2.3). Only cis- 1 -phenylcyclo-
hexene is produced. Likewise, as Equation 2.3 shows, cis-2-phenylcyclohexyl
tosylate also loses TsOH stereoselectively to form cis-1-phenylcyclohexene.
In a stereospecijic reaction diastereomerically different starting materials give
diastereomerically different products. Thus the bromination of the 2-butenes
(Equations 2.1 and 2.2) is stereospecific, since one geometrical isomer gives one
product and the other isomer a diastereometrically different product. Elimination
ofTsOH from the two 2-phenylcyclohexyl tosylates, however, is not stereospecific.