Page 79 - Mechanism and Theory in Organic Chemistry
P. 79
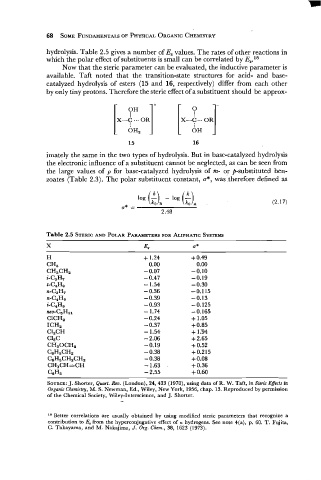
hydrolysis. Table 2.5 gives a number of Es values. The rates of other reactions in
which the polar effect of substituents is small can be correlated by ES.l8
Now that the steric parameter can be evaluated, the inductive parameter is
available. Taft noted that the transition-state structures for acid- and base-
catalyzed hydrolysis of esters (15 and 16, respectively) differ from each other
by only tiny protons. Therefore the steric effect of a substituent should be approx-
imately the same in the two types of hydrolysis. But in base-catalyzed hydrolysis
the electronic influence of a substituent cannot be neglected, as can be seen from
the large values of p for base-catalyzed hydrolysis of m- or p-substituted ben-
zoates (Table 2.3). The polar substituent constant, a*, was .therefore defined as
Table 2.5 STERIC AND POLAR PARAMETERS FOR ALIPHATIC SYSTEMS
X E8 u*
H + 1.24 + 0.49
CH, 0.00 0.00
CH3CHa - 0.07 -0.10
i-C3H7 - 0.47 -0.19
t-C4He - 1.54 - 0.30
n-C3H7 - 0.36 -0.115
n-C4H9 - 0.39 - 0.13
i-C4He - 0.93 - 0.125
neo-C5Hll - 1.74 - 0.165
ClCH, - 0.24 + 1.05
ICH, - 0.37 + 0.85
C12CH - 1.54 + 1.94
C13C -2.06 + 2.65
CH30CHa -0.19 + 0.52
ceH,CHz - 0.38 +0.215
CeH5CHzCH2 - 0.38 + 0.08
CH3CH=CH - 1.63 + 0.36
CeH, - 2.55 + 0.60
SOURCE: J. Shorter, Quart. Rev. (London), 24, 433 (1970), using data of R. W. Taft, in Sfcric Effects in
Organic Chemistry, M. S. Newrnan, Ed., Wiley, New York, 1956, chap. 13. Reproduced by permission
of the Chemical Society, Wiley-Interscience, and J. Shorter.
-
la Better correlations are usually obtained by using modified steric parameters that recognize a
contribution to E, from the hyperconjugative effect of a hydrogens. See note 4(a), p. 60. T. Fujita,
C. Takayarna, and M. Nakajima, J. Org. Chem., 38, 1623 (1973).