Page 81 - Mechanism and Theory in Organic Chemistry
P. 81
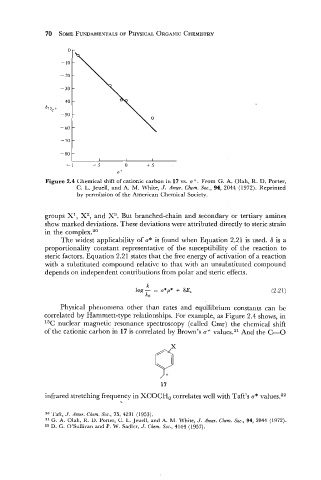
Figure 2.4 Chemical shift of cationic carbon in 17 vs. a+. From G. A. Olah, R. D. Porter,
C. L. Jeuell, and A. M. White, J. Amer. Chem. Soc., 94, 2044 (1972). Reprinted
by permission of the American Chemical Society.
groups X1, X2, and X3. Rut branched-chain and secondary or tertiary amines
show marked deviations. These deviations were attributed directly to steric strain
in the complex.20
The widest applicability of a* is found when Equation 2.21 is used. 6 is a
proportionality constant representative of the susceptibility of the reaction to
steric factors. Equation 2.21 states that the free energy of activation of a reaction
with a substituted compound relative to that with an unsubstituted compound
depends on independent contributions from polar and steric effects.
k
log - = + 6E, (2.21)
ko
Physical phenomena other than rates and equilibrium constants can be
correlated by Hammett-type relationships. For example, as Figure 2.4 shows, in
13C nuclear magnetic resonance spectroscopy (called Cmr) the chemical shift
of the cationic carbon in 17 is correlated by Brown's a+ values.21 And the C=O
infrared stretching frequency in XCOCH, correlates well with Taft's a* values.22
Taft, J. Amer. Chem. Sac., 75. 4231 (1953).
G. A. Olah, R. D. Porter, C. L. Jcuell, and A. hl. White, .J. Amer. Chem. Sac., 94, 2044 (1972).
ZZ D. G. O'Sullivan and P. W. Sadler, J. Chem. Sac., 4144 (1957).