Page 83 - Mechanism and Theory in Organic Chemistry
P. 83
hydrogens. By Benson's notation this group is designated C-(H),(C) : the central
atom in the group is given first and then the ligand atoms in parentheses. AHhf
each group is calculated from experimentally determined AH,"'s of compounds
that contain that group. Then AH," for a new molecule in the gas phase is obtained
by simply adding together the contributions from each group. AH; for the
C-(H),(C) group is - 10.08 kcal mole-l). Thus ethane is calculated to have a
AH>f - 20.16 kcal mole-l. Propane also has two C-(H),(C) groups and a
C-(H),(C), group (AH; = - 4.95 kcal mole- I). Therefore AH; (CH,CH,CH,)
= -20.16 - 4.95 = - 25.1 1 kcal mole-l. The experimental AH/"'s for ethane
and propane are - 20.24 and - 24.82 kcal mole - l, respectively. Benson's addi-
tivity rules do not apply to condensed-phase compounds because of the contri-
bution of solvation and of lattice and hydrogen bond energies to AH,O(X) in the
liquid and solid phases. These contributions are, of course, not additive.
Tables 2.6 through 2.10 (see pp. 75-83) list AH/" values for a large number
of groups (see Section 9.1 for additivity data for radicals). In these tables, Cd
refers to a carbon that is forming a carbon-carbon double bond. The notation
Cd-(H),(Cd) is shortened to Cd-(H,), since all carbon-carbon double bonds are
between two sp2 carbons. Similarly, C,-(X) refers to a carbon triply bonded to
another sp carbon and to an X ligand; C,-(X) refers to an aromatic ring carbon
bonded to two other ring carbons and to a substituent X; and C, refers to the
central carbon of the allenic group C=C=C. Other group abbreviations are
noted at the end of the appropriate table.
In simply adding together the AH,"'s of all the groups in a molecule to ob-
tain the AH/" of the molecule, we make the assumption that only the nearest
neighbors of a bond affect that bond. This is not always true, and we shall now
discuss the more important corrections that must be applied if the group additivity
scheme for molecular enthalpies is to be used successfully.
Alkanes
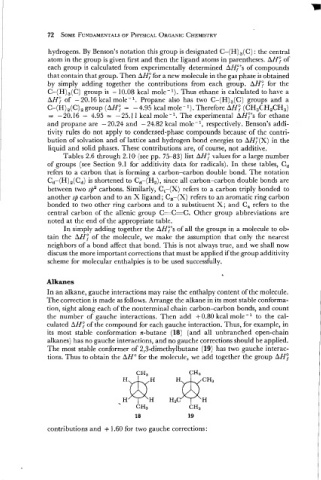
In an alkane, gauche interactions may raise the enthalpy content of the molecule.
The correction is made as follows. Arrange the alkane in its most stable conforma-
tion, sight along each of the nonterminal chain carbon-carbon bonds, and count
the number of gauche interactions. Then add f0.80 kcal mole-l to the cal-
culated AH/" of the compound for each gauche interaction. Thus, for example, in
its most stable conformation n-butane (18) (and all unbranched open-chain
alkanes) has no gauche interactions, and no gauche corrections should be applied.
The most stable conformer of 2,3-dimethylbutane (19) has two gauche interac-
tions. Thus to obtain the AH0 for the molecule, we add together the group AH;
contributions and + 1.60 for two gauche corrections :