Page 78 - Mechanism and Theory in Organic Chemistry
P. 78
Linear Free-Energy Relationships 67
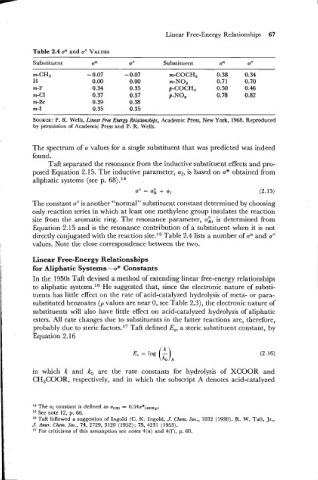
Table 2.4 on and o0 VALUES
Substituent on u0 Substituent un u0
m-CH, - 0.07 - 0.07 m-COCH, 0.38 0.34
H 0.00 0.00 m-NO2 0.71 0.70
m-F 0.34 0.35 p-COCH, 0.50 0.46
m-C1 0.37 0.37 p-NO2 0.78 0.82
m-Br 0.39 0.38
m-I 0.35 0.35
P.
SOURCE: R. Wells, Linear Free Energy Relationships, Academic Press, New York, 1968. Reproduced
by permission of Academic Press and P. R. Wells.
The spectrum of u values for a single substituent that was predicted was indeed
found.
Taft separated the resonance from the inductive substituent effects and pro-
posed Equation 2.15. The inductive parameter, a,, is based on a* obtained from
aliphatic systems (see p. 68).14
o0 = og + u1 (2.15)
The constant o0 is another "normal" substituent constant determined by choosing
only reaction series in which at least one methylene group insulates the reaction
site from the aromatic ring. The resonance parameter, a;, is determined from
Equation 2.15 and is the resonance contribution of a substituent when it is not
directly conjugated with the reaction site.15 Table 2.4 lists a number of an and u0
values. Note the close correspondence between the two.
Linear Free-Energy Relationships
for Aliphatic Systems-u* Constants
In the 1950s Taft devised a method of extending linear free-energy relationships
to aliphatic systems.16 He suggested that, since the electronic nature of substi-
tuents has little effect on the rate of acid-catalyzed hydrolysis of meta- or para-
substituted benzoates (p values are near 0, see Table 2.3), the electronic nature of
substituents will also have little effect on acid-catalyzed hydrolysis of aliphatic
esters. All rate changes due to substituents in the latter reactions are, therefore,
probably due to steric factors.17 Taft defined E,, a steric substituent constant, by
Equation 2.16
in which k and k, are the rate constants for hydrolysis of XCOOR and
CH,COOR, respectively, and in which the subscript A denotes acid-catalyzed
l4 The a1 constant is defined as alcx, = 0.54~*(xc~~,.
l5 See note 12, p. 66.
l6 Taft followed a suggestion of Ingold (C. K. Ingold, J. Chem. Soc., 1032 (1930). R. W. Taft, Jr.,
J. Amer. Chem. Soc., 74, 2729, 3120 (1952) ; 75, 4231 (1953).
l7 For criticisms of this assumption see notes 4(a) and 4(f), p. 60.