Page 43 - Methods For Monitoring And Diagnosing The Efficiency Of Catalytic Converters A Patent - oriented Survey
P. 43
Introduction 25
Monitoring and diagnosing the efJiciency of catalytic converters
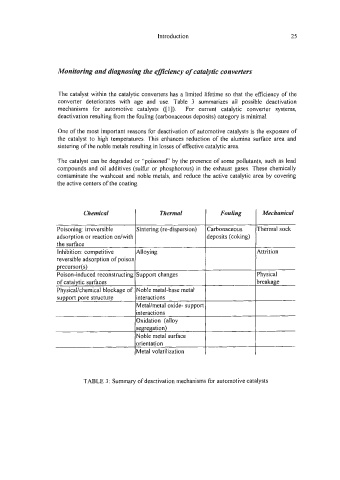
The catalyst within the catalytic converters has a limited lifetime so that the efficiency of the
converter deteriorates with age and use. Table 3 summarizes all possible deactivation
mechanisms for automotive catalysts ([ 11). For current catalytic converter systems,
deactivation resulting from the fouling (carbonaceous deposits) category is minimal.
One of the most important reasons for deactivation of automotive catalysts is the exposure of
the catalyst to high temperatures. This enhances reduction of the alumina surface area and
sintering of the noble metals resulting in losses of effective catalytic area.
The catalyst can be degraded or “poisoned by the presence of some pollutants, such as lead
compounds and oil additives (sulfir or phosphorous) in the exhaust gases. These chemically
contaminate the washcoat and noble metals, and reduce the active catalytic area by covering
the active centers of the coating.
I I I
Chemical
Poisoning: irreversible Sintering (re-dispersion) Carbonaceous Thermal sock
adsorption or reaction odwith deposits (coking)
the surface
Inhibition: competitive Alloying Attrition
reversible adsorption of poisof
precursor(s)
Poison-induced reconstructing Support changes Physicai
of catalytic surfaces breakage
Physical/chemical blockage of INoble metal-base metal
support pore structure linteractions I
(MetaVmetal oxide- support
linteractions I I
IOxidation (alloy
segregation)
Noble metal surface
orientation
Metal volatilization
TABLE 3: Summary of deactivation mechanisms for automotive catalysts