Page 104 - Modeling of Chemical Kinetics and Reactor Design
P. 104
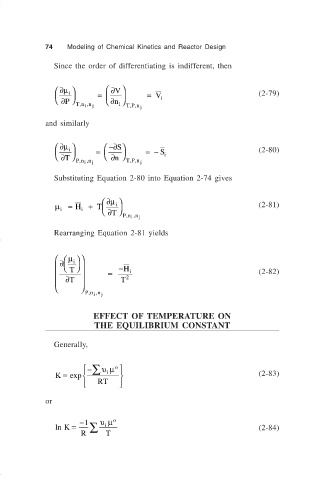
74 Modeling of Chemical Kinetics and Reactor Design
Since the order of differentiating is indifferent, then
∂
∂µ V
i = = V i (2-79)
∂
∂
P n
,
i ,
,,
Tn n j i TPn j
and similarly
∂µ i −∂ S
= =− S i (2-80)
∂
∂
T n ,,
i ,
,
Pn n j TPn j
Substituting Equation 2-80 into Equation 2-74 gives
∂ µ
µ = H + T i (2-81)
i i ∂ T
i ,
,
Pn n j
Rearranging Equation 2-81 yields
∂ µ i
T −H i (2-82)
= 2
∂T T
,
i ,
Pn n j
EFFECT OF TEMPERATURE ON
THE EQUILIBRIUM CONSTANT
Generally,
∑ υµ o
−
K = exp i (2-83)
RT
or
ln K = −1 ∑ υµ o (2-84)
i
R T