Page 105 - Modeling of Chemical Kinetics and Reactor Design
P. 105
Thermodynamics of Chemical Reactions 75
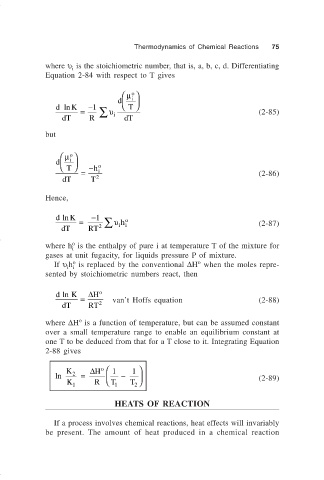
where υ is the stoichiometric number, that is, a, b, c, d. Differentiating
i
Equation 2-84 with respect to T gives
µ o
d i
d ln K −1 T
= ∑ υ (2-85)
dT R i dT
but
µ
o
d i
T − h o
= i (2-86)
dT T 2
Hence,
d ln K −1
= ∑ υ h o (2-87)
dT RT 2 ii
o
where h is the enthalpy of pure i at temperature T of the mixture for
i
gases at unit fugacity, for liquids pressure P of mixture.
o
o
If υ h is replaced by the conventional ∆H when the moles repre-
i i
sented by stoichiometric numbers react, then
d ln K ∆ H o
= van’t Hoffs equation (2-88)
dT RT 2
o
where ∆H is a function of temperature, but can be assumed constant
over a small temperature range to enable an equilibrium constant at
one T to be deduced from that for a T close to it. Integrating Equation
2-88 gives
o
K 2 ∆ H 1 1
ln = − (2-89)
K 1 R T 1 T
2
HEATS OF REACTION
If a process involves chemical reactions, heat effects will invariably
be present. The amount of heat produced in a chemical reaction