Page 134 - Modeling of Chemical Kinetics and Reactor Design
P. 134
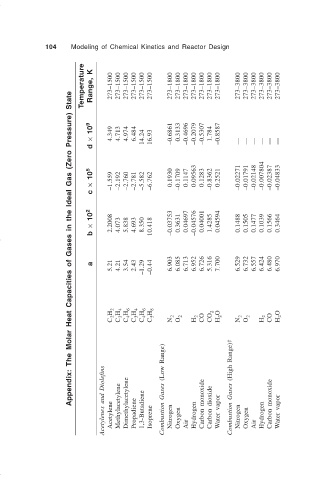
104 Modeling of Chemical Kinetics and Reactor Design
Temperature Range, K 273–1500 273–1500 273–1500 273–1500 273–1500 273–1500 273–1800 273–1800 273–1800 273–1800 273–1800 273–1800 273–1800 273–3800 273–3800 273–3800 273–3800 273–3800 273–3800
Appendix: The Molar Heat Capacities of Gases in the Ideal Gas (Zero Pressure) State
d × 10 9 4.349 4.713 4.974 6.484 14.24 16.93 –0.6861 0.3133 –0.4696 –0.2079 –0.5307 1.784 –0.8587 — — — — — —
c × 10 5 –1.559 –2.192 –2.760 –2.781 –5.582 –6.762 0.1930 –0.1709 0.1147 0.09563 0.1283 –0.8362 0.2521 –0.02271 –0.01791 –0.02148 –0.007804 –0.02387 –0.04833
× 10 2 2.2008 4.073 5.838 4.693 8.350 –0.03753 0.3631 0.04697 –0.04576 0.04001 1.4285 0.04594 0.1488 0.1505 0.1477 0.1039 0.1566 0.3464
b 10.418
5.21 4.21 3.54 2.43 –1.29 –0.44
a 6.903 6.085 6.713 6.952 6.726 5.316 7.700 6.529 6.732 6.557 6.424 6.480 6.970
C 2 H 2 C 3 H 4 C 4 H 6 C 3 H 4 C 4 H 6 C 5 H 8 N 2 O 2 H 2 CO CO 2 H 2 O N 2 O 2 H 2 CO H 2 O
Acetylenes and Diolefins Acetylene Methylacetylene Dimethylacetylene Propadiene 1,3-Butadiene Isoprene Combustion Gases (Low Range) Nitrogen Oxygen Air Hydrogen Carbon monoxide Carbon dioxide Water vapor Combustion Gases (High Range)† Nitrogen Oxygen Air Hydrogen Carbon monoxide Water vapor