Page 295 - Modeling of Chemical Kinetics and Reactor Design
P. 295
Introduction to Reactor Design Fundamentals for Ideal Systems 265
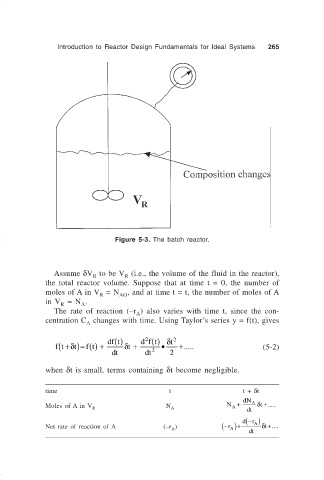
Figure 5-3. The batch reactor.
Assume δV to be V (i.e., the volume of the fluid in the reactor),
R
R
the total reactor volume. Suppose that at time t = 0, the number of
moles of A in V = N AO , and at time t = t, the number of moles of A
R
in V = N .
R
A
The rate of reaction (–r ) also varies with time t, since the con-
A
centration C changes with time. Using Taylor’s series y = f(t), gives
A
2
(
f t)
ft +δ t)= ( + df t ( ) δ t + df t ( ) δ t 2 +..... (5-2)
•
dt dt 2 2
when δt is small, terms containing δt become negligible.
time t t + δt
N + dN A δ
Moles of A in V R N A A dt t +.....
− ( dr A )
Net rate of reaction of A (–r ) − ( r A )+ + t δ ....
A
dt