Page 298 - Modeling of Chemical Kinetics and Reactor Design
P. 298
268 Modeling of Chemical Kinetics and Reactor Design
C AF dC
t =− ∫ r − ( A ) =, for ε = 0 (5-17)
A
C AO
Equations 5-12, 5-16, and 5-17 are the performance equations for
a batch reactor.
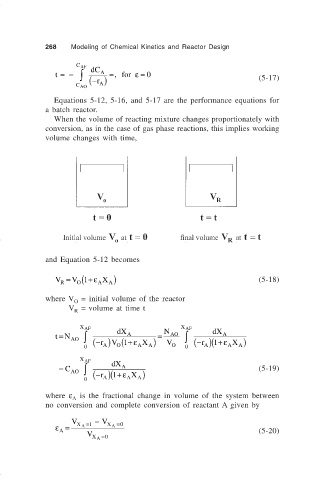
When the volume of reacting mixture changes proportionately with
conversion, as in the case of gas phase reactions, this implies working
volume changes with time,
and Equation 5-12 becomes
V = V (1 ε X ) (5-18)
+
R O A A
where V = initial volume of the reactor
O
V = volume at time t
R
X AF dX N X AF dX
=
A
tN AO ∫ r − ( V ) (1 ε X ) = AO ∫ r − ( A X )
+
0 A O A A V O 0 A ) + (1 ε A A
X AF
= C AO ∫ r − ( dX A X ) (5-19)
0 A ) + (1 ε A A
where ε is the fractional change in volume of the system between
A
no conversion and complete conversion of reactant A given by
V X A =1 − V X A =0
ε =
A (5-20)
V X A =0