Page 300 - Modeling of Chemical Kinetics and Reactor Design
P. 300
270 Modeling of Chemical Kinetics and Reactor Design
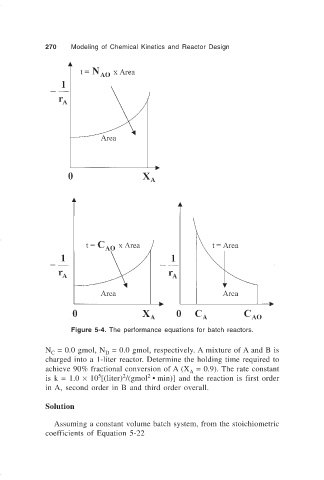
Figure 5-4. The performance equations for batch reactors.
N = 0.0 gmol, N = 0.0 gmol, respectively. A mixture of A and B is
D
C
charged into a 1-liter reactor. Determine the holding time required to
achieve 90% fractional conversion of A (X = 0.9). The rate constant
A
5
2
2
is k = 1.0 × 10 [(liter) /(gmol • min)] and the reaction is first order
in A, second order in B and third order overall.
Solution
Assuming a constant volume batch system, from the stoichiometric
coefficients of Equation 5-22