Page 324 - Modeling of Chemical Kinetics and Reactor Design
P. 324
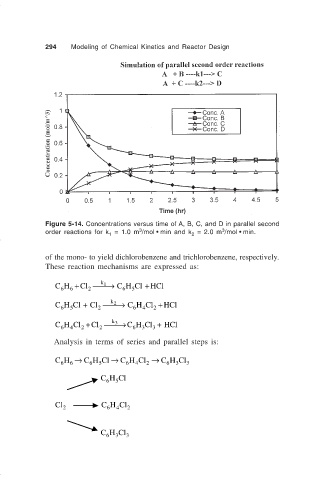
294 Modeling of Chemical Kinetics and Reactor Design
Figure 5-14. Concentrations versus time of A, B, C, and D in parallel second
3
3
order reactions for k = 1.0 m /mol • min and k = 2.0 m /mol • min.
1
2
of the mono- to yield dichlorobenzene and trichlorobenzene, respectively.
These reaction mechanisms are expressed as:
C H + Cl → C H Cl + HCl
k 1
6
2
6
5
6
C H Cl + Cl → C H Cl + HCl
k 2
6 5 2 6 4 2
C H Cl + Cl → C H Cl + HCl
k 3
3
3
6
2
2
6
4
Analysis in terms of series and parallel steps is:
CH → CH Cl → CH Cl → CH Cl 3
4
6
3
6
2
6
5
6
6
CH Cl
6 5
Cl CH Cl
2 6 4 2
CH Cl 3
3
6