Page 321 - Modeling of Chemical Kinetics and Reactor Design
P. 321
Introduction to Reactor Design Fundamentals for Ideal Systems 291
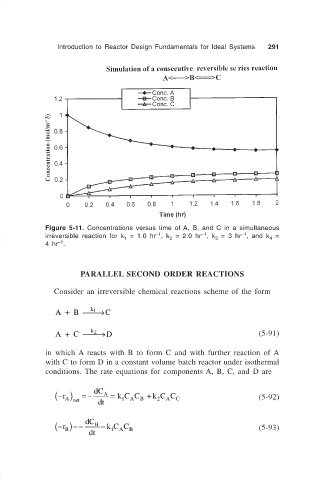
Figure 5-11. Concentrations versus time of A, B, and C in a simultaneous
–1
–1
–1
irreversible reaction for k = 1.0 hr , k = 2.0 hr , k = 3 hr , and k =
4
3
1
2
–1
4 hr .
PARALLEL SECOND ORDER REACTIONS
Consider an irreversible chemical reactions scheme of the form
A + B → C
k 1
A + C → D (5-91)
k 2
in which A reacts with B to form C and with further reaction of A
with C to form D in a constant volume batch reactor under isothermal
conditions. The rate equations for components A, B, C, and D are
− ( r ) =− dC A = kC C + k C C (5-92)
A net 1 A B 2 A C
dt
− ( r )=− dC B = kC C (5-93)
B 1 A B
dt