Page 317 - Modeling of Chemical Kinetics and Reactor Design
P. 317
Introduction to Reactor Design Fundamentals for Ideal Systems 287
–1
and k = 0.75 hr for a period of 5 hours with time increment h = ∆t
2
–1
= 0.5 hr . Using the developed Runge-Kutta fourth order computer
program BATCH52, the concentrations of A, B, and C can be predicted
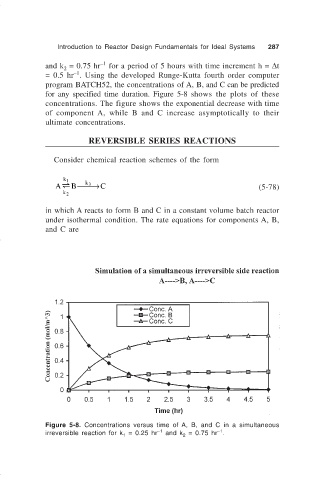
for any specified time duration. Figure 5-8 shows the plots of these
concentrations. The figure shows the exponential decrease with time
of component A, while B and C increase asymptotically to their
ultimate concentrations.
REVERSIBLE SERIES REACTIONS
Consider chemical reaction schemes of the form
A[ B → C (5-78)
k 1
k 3
k 2
in which A reacts to form B and C in a constant volume batch reactor
under isothermal condition. The rate equations for components A, B,
and C are
Figure 5-8. Concentrations versus time of A, B, and C in a simultaneous
–1
–1
irreversible reaction for k = 0.25 hr and k = 0.75 hr .
2
1