Page 315 - Modeling of Chemical Kinetics and Reactor Design
P. 315
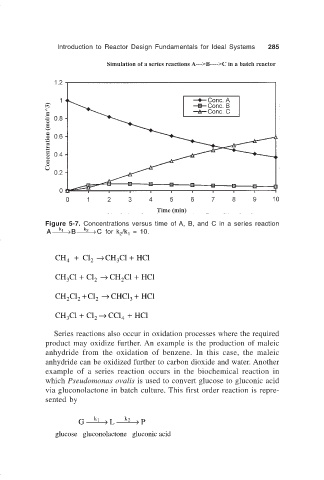
Introduction to Reactor Design Fundamentals for Ideal Systems 285
Figure 5-7. Concentrations versus time of A, B, and C in a series reaction
A → B → for k /k = 10.
C
k 2
k 1
2 1
CH 4 + Cl → CH Cl + HCl
2
3
CH Cl + Cl → CH Cl + HCl
3
2
2
CH Cl + Cl → CHCl + HCl
2 2 2 3
CH Cl + Cl → CCl + HCl
3
4
2
Series reactions also occur in oxidation processes where the required
product may oxidize further. An example is the production of maleic
anhydride from the oxidation of benzene. In this case, the maleic
anhydride can be oxidized further to carbon dioxide and water. Another
example of a series reaction occurs in the biochemical reaction in
which Pseudomonas ovalis is used to convert glucose to gluconic acid
via gluconolactone in batch culture. This first order reaction is repre-
sented by
G → L → P
k 1
k 2
glucose gluconolactone gluconic acid