Page 54 - Modeling of Chemical Kinetics and Reactor Design
P. 54
24 Modeling of Chemical Kinetics and Reactor Design
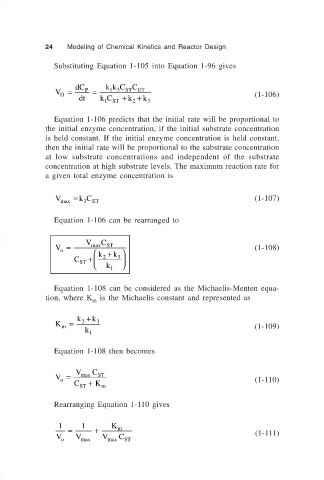
Substituting Equation 1-105 into Equation 1-96 gives
V = dC P = kk C C ET (1-106)
ST
13
O
dt kC + k + k
1 ST 2 3
Equation 1-106 predicts that the initial rate will be proportional to
the initial enzyme concentration, if the initial substrate concentration
is held constant. If the initial enzyme concentration is held constant,
then the initial rate will be proportional to the substrate concentration
at low substrate concentrations and independent of the substrate
concentration at high substrate levels. The maximum reaction rate for
a given total enzyme concentration is
V max = k C ET (1-107)
3
Equation 1-106 can be rearranged to
C
V = V max k + k (1-108)
ST
o
+
C ST 2 3
k 1
Equation 1-108 can be considered as the Michaelis-Menten equa-
tion, where K is the Michaelis constant and represented as
m
k + k
K = 2 3
m (1-109)
k 1
Equation 1-108 then becomes
V = V max C ST
o (1-110)
C ST + K m
Rearranging Equation 1-110 gives
1 1 K
= + m
V V V C (1-111)
o max max ST