Page 76 - Modeling of Chemical Kinetics and Reactor Design
P. 76
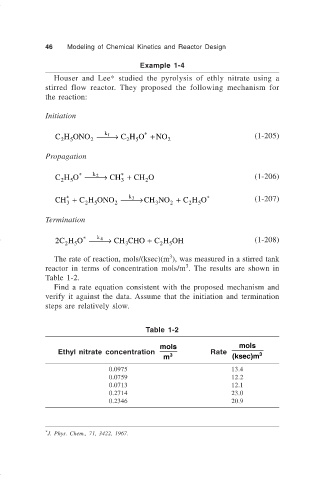
46 Modeling of Chemical Kinetics and Reactor Design
Example 1-4
Houser and Lee* studied the pyrolysis of ethly nitrate using a
stirred flow reactor. They proposed the following mechanism for
the reaction:
Initiation
CH ONO → CH O + NO 2 (1-205)
*
k 1
2
2
5
5
2
Propagation
CH O → CH + CHO (1-206)
*
*
k 2
5
3
2
2
CH + C H ONO → CH NO + C H O * (1-207)
*
k 3
2
5
3
2
2
3
5
2
Termination
2C H O → CH CHO + C H OH (1-208)
k
*
4
2 5 3 2 5
3
The rate of reaction, mols/(ksec)(m ), was measured in a stirred tank
3
reactor in terms of concentration mols/m . The results are shown in
Table 1-2.
Find a rate equation consistent with the proposed mechanism and
verify it against the data. Assume that the initiation and termination
steps are relatively slow.
Table 1-2
mols mols
Ethyl nitrate concentration Rate 3
m 3 (ksec)m
0.0975 13.4
0.0759 12.2
0.0713 12.1
0.2714 23.0
0.2346 20.9
* J. Phys. Chem., 71, 3422, 1967.