Page 81 - Modeling of Chemical Kinetics and Reactor Design
P. 81
Reaction Mechanisms and Rate Expressions 51
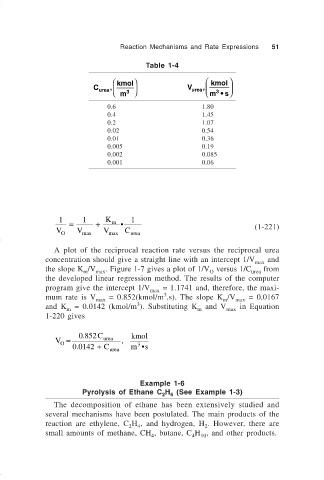
Table 1-4
kmol kmol
C urea 3 V urea 3
,
,
m
m •s
0.6 1.80
0.4 1.45
0.2 1.07
0.02 0.54
0.01 0.36
0.005 0.19
0.002 0.085
0.001 0.06
1 1 K 1
= + m •
V V V C (1-221)
O max max urea
A plot of the reciprocal reaction rate versus the reciprocal urea
concentration should give a straight line with an intercept 1/V and
max
the slope K /V . Figure 1-7 gives a plot of 1/V versus 1/C from
m max O urea
the developed linear regression method. The results of the computer
program give the intercept 1/V = 1.1741 and, therefore, the maxi-
max
3
mum rate is V = 0.852(kmol/m .s). The slope K /V = 0.0167
max m max
3
and K = 0.0142 (kmol/m ). Substituting K and V in Equation
m m max
1-220 gives
.
V = 0 852 C urea , kmol
O
3
0 0142 + C urea m • s
.
Example 1-6
Pyrolysis of Ethane C H (See Example 1-3)
2
6
The decomposition of ethane has been extensively studied and
several mechanisms have been postulated. The main products of the
reaction are ethylene, C H , and hydrogen, H . However, there are
2 4 2
small amounts of methane, CH , butane, C H , and other products.
4 4 10