Page 79 - Modeling of Chemical Kinetics and Reactor Design
P. 79
Reaction Mechanisms and Rate Expressions 49
.
− ( r ) = kC 05 (1-218)
2
5
5
C H ONO 2 net C H ONO 2
2
B
Equation 1-218 can be expressed in the form Y = AX .
B
Using natural logarithm, Equation Y = AX gives
ln Y ( ) = ln A B •ln X ( ) (1-219)
+
If we perform a regression analysis on Equation 1-219, the slope
gives the order of the reaction and the rate constant is determined by
the intercept. Appendix A illustrates a developed computer program
that performs the regression analysis of equations for any given set
of data. The results of the linearized regression analysis from the
computer program give slope B as 0.486, and intercept A as 42.7.
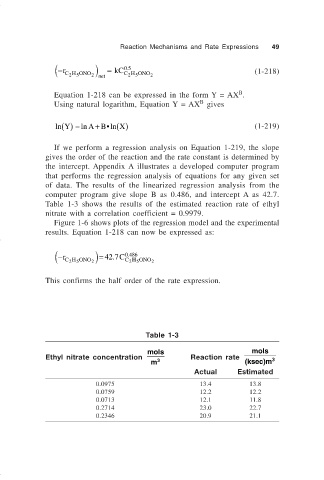
Table 1-3 shows the results of the estimated reaction rate of ethyl
nitrate with a correlation coefficient = 0.9979.
Figure 1-6 shows plots of the regression model and the experimental
results. Equation 1-218 can now be expressed as:
.
− ( r ) = 42 7. C 0 486
2
5
5
2
C H ONO 2 C H ONO 2
This confirms the half order of the rate expression.
Table 1-3
mols mols
Ethyl nitrate concentration Reaction rate 3
m 3 (ksec)m
Actual Estimated
0.0975 13.4 13.8
0.0759 12.2 12.2
0.0713 12.1 11.8
0.2714 23.0 22.7
0.2346 20.9 21.1