Page 82 - Modeling of Chemical Kinetics and Reactor Design
P. 82
52 Modeling of Chemical Kinetics and Reactor Design
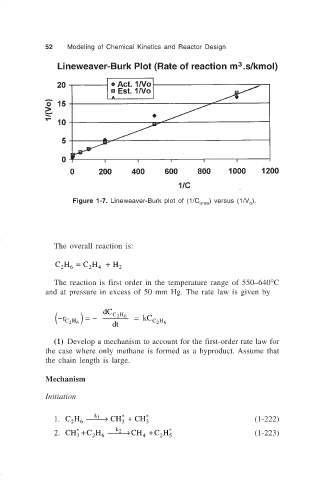
Figure 1-7. Lineweaver-Burk plot of (1/C urea ) versus (1/V ).
o
The overall reaction is:
CH = CH + H
2 6 2 4 2
The reaction is first order in the temperature range of 550–640°C
and at pressure in excess of 50 mm Hg. The rate law is given by
dC
2
− ( r ) =− CH 6 = kC
CH 6 dt CH 6
2
2
(1) Develop a mechanism to account for the first-order rate law for
the case where only methane is formed as a byproduct. Assume that
the chain length is large.
Mechanism
Initiation
1. C H → CH + CH * 3 (1-222)
*
k 1
3
2
6
2. CH + C H → CH + C H * 5 (1-223)
*
k 2
2
4
6
2
3