Page 83 - Modeling of Chemical Kinetics and Reactor Design
P. 83
Reaction Mechanisms and Rate Expressions 53
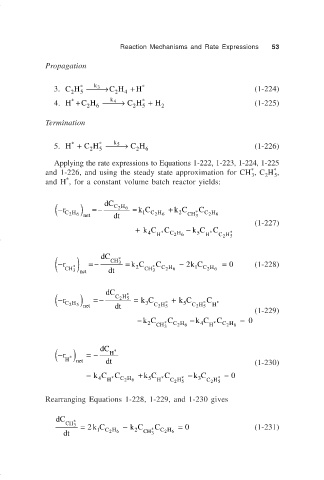
Propagation
3. CH → CH + H * (1-224)
*
k 3
4
2
5
2
4. H + C H → C H + H 2 (1-225)
*
*
k 4
6
2
2
5
Termination
5. H + C H → C H 6 (1-226)
*
*
k 5
5
2
2
Applying the rate expressions to Equations 1-222, 1-223, 1-224, 1-225
*
*
and 1-226, and using the steady state approximation for CH , C H ,
3
5
2
*
and H , for a constant volume batch reactor yields:
) dC CH 6
2
− ( r =− = kC + k C C
2
CH 6 net dt 1 CH 6 2 CH 3 * CH 6
2
2
(1-227)
+ kC H * C CH 6 − k C H * C C H 5 *
4
5
2
2
( −r ) =− dC CH 3 * = kC C − k C = 0 (1-228)
2
2
2
CH 3 * dt 2 CH 3 * CH 6 1 CH 6
net
2
− ( r ) =− dC CH 5 * = kC + k C C
2
CH 5 net dt 3 CH 5 * 5 CH 5 * H *
2
2
(1-229)
− kC * C − k C * C = 0
2
4
2
2
CH 3 CH 6 H CH 6
( −r H * ) net =− dC H * (1-230)
dt
= kC H * C CH 6 + k C H * C C H 5 * − k C C H 5 * = 0
4
3
5
2
2
2
Rearranging Equations 1-228, 1-229, and 1-230 gives
dC *
= 2 kC − k C C = 0 (1-231)
CH 3
2
2
dt 1 CH 6 2 CH 3 * CH 6