Page 117 - Modern Analytical Chemistry
P. 117
1400-CH04 9/8/99 3:55 PM Page 100
100 Modern Analytical Chemistry
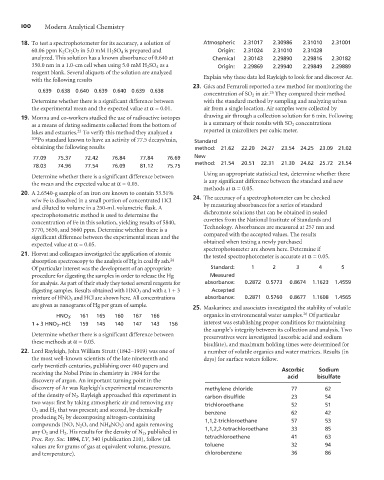
18. To test a spectrophotometer for its accuracy, a solution of Atmospheric 2.31017 2.30986 2.31010 2.31001
60.06 ppm K 2 Cr 2 O 7 in 5.0 mM H 2 SO 4 is prepared and Origin: 2.31024 2.31010 2.31028
analyzed. This solution has a known absorbance of 0.640 at Chemical 2.30143 2.29890 2.29816 2.30182
350.0 nm in a 1.0-cm cell when using 5.0 mM H 2 SO 4 as a Origin: 2.29869 2.29940 2.29849 2.29889
reagent blank. Several aliquots of the solution are analyzed
Explain why these data led Rayleigh to look for and discover Ar.
with the following results
23. Gács and Ferraroli reported a new method for monitoring the
0.639 0.638 0.640 0.639 0.640 0.639 0.638 25
concentration of SO 2 in air. They compared their method
Determine whether there is a significant difference between with the standard method by sampling and analyzing urban
the experimental mean and the expected value at a= 0.01. air from a single location. Air samples were collected by
19. Monna and co-workers studied the use of radioactive isotopes drawing air through a collection solution for 6 min. Following
as a means of dating sediments collected from the bottom of is a summary of their results with SO 2 concentrations
23
lakes and estuaries. To verify this method they analyzed a reported in microliters per cubic meter.
208 Po standard known to have an activity of 77.5 decays/min, Standard
obtaining the following results method: 21.62 22.20 24.27 23.54 24.25 23.09 21.02
77.09 75.37 72.42 76.84 77.84 76.69 New
method: 21.54 20.51 22.31 21.30 24.62 25.72 21.54
78.03 74.96 77.54 76.09 81.12 75.75
Using an appropriate statistical test, determine whether there
Determine whether there is a significant difference between
is any significant difference between the standard and new
the mean and the expected value at a= 0.05.
methods at a= 0.05.
20. A 2.6540-g sample of an iron ore known to contain 53.51%
24. The accuracy of a spectrophotometer can be checked
w/w Fe is dissolved in a small portion of concentrated HCl
by measuring absorbances for a series of standard
and diluted to volume in a 250-mL volumetric flask. A
dichromate solutions that can be obtained in sealed
spectrophotometric method is used to determine the
cuvettes from the National Institute of Standards and
concentration of Fe in this solution, yielding results of 5840,
Technology. Absorbances are measured at 257 nm and
5770, 5650, and 5660 ppm. Determine whether there is a
compared with the accepted values. The results
significant difference between the experimental mean and the
obtained when testing a newly purchased
expected value at a= 0.05.
spectrophotometer are shown here. Determine if
21. Horvat and colleagues investigated the application of atomic the tested spectrophotometer is accurate at a= 0.05.
absorption spectroscopy to the analysis of Hg in coal fly ash. 24
Of particular interest was the development of an appropriate Standard: 1 2 3 4 5
procedure for digesting the samples in order to release the Hg Measured
for analysis. As part of their study they tested several reagents for absorbance: 0.2872 0.5773 0.8674 1.1623 1.4559
digesting samples. Results obtained with HNO 3 and with a 1 + 3 Accepted
mixture of HNO 3 and HCl are shown here. All concentrations absorbance: 0.2871 0.5760 0.8677 1.1608 1.4565
are given as nanograms of Hg per gram of sample.
25. Maskarinec and associates investigated the stability of volatile
26
HNO 3 : 161 165 160 167 166 organics in environmental water samples. Of particular
1 + 3 HNO 3 –HCl: 159 145 140 147 143 156 interest was establishing proper conditions for maintaining
the sample’s integrity between its collection and analysis. Two
Determine whether there is a significant difference between
preservatives were investigated (ascorbic acid and sodium
these methods at a= 0.05.
bisulfate), and maximum holding times were determined for
22. Lord Rayleigh, John William Strutt (1842–1919) was one of a number of volatile organics and water matrices. Results (in
the most well-known scientists of the late nineteenth and days) for surface waters follow.
early twentieth centuries, publishing over 440 papers and
Ascorbic Sodium
receiving the Nobel Prize in chemistry in 1904 for the
acid bisulfate
discovery of argon. An important turning point in the
discovery of Ar was Rayleigh’s experimental measurements methylene chloride 77 62
of the density of N 2. Rayleigh approached this experiment in carbon disulfide 23 54
two ways: first by taking atmospheric air and removing any trichloroethane 52 51
O 2 and H 2 that was present; and second, by chemically
benzene 62 42
producing N 2 by decomposing nitrogen-containing
1,1,2-trichloroethane 57 53
compounds (NO, N 2 O, and NH 4 NO 3 ) and again removing
1,1,2,2-tetrachloroethane 33 85
any O 2 and H 2 . His results for the density of N 2 , published in
tetrachloroethene 41 63
Proc. Roy. Soc. 1894, LV, 340 (publication 210), follow (all
values are for grams of gas at equivalent volume, pressure, toluene 32 94
and temperature). chlorobenzene 36 86