Page 224 - Modern Analytical Chemistry
P. 224
1400-CH07 9/8/99 4:03 PM Page 207
Chapter 7 Obtaining and Preparing Samples for Analysis 207
7 5
Table . Conditions for the Separation of Selected Cellular
Components by Centrifugation
Centrifugal Force Time
Components (´g) (min)
eukaryotic cell 1000 5
cell membranes, nuclei 4000 10
mitochondria, bacterial cells 15,000 20
lysosomes, bacterial membranes 30,000 30
ribosomes 100,000 180
Source: Adapted from Zubay G. Biochemistry, 2nd ed. Macmillan: New York, 1988, p. 120.
centrifuge tube. For particles of equal density the separation is based on mass, with
heavier particles having greater sedimentation rates. When the particles are of equal
mass, those with the highest density have the greatest sedimentation rate.
Centrifugation is of particular importance as a separation technique in biochem-
istry. As shown in Table 7.5, cellular components can be separated by centrifugation. 12
For example, lysosomes can be separated from other cellular components by repeated
differential centrifugation, in which the sample is divided into a solid residue and a so-
lution called the supernatant. After destroying the cell membranes, the solution
is centrifuged at 15,000 ´g (a centrifugal field strength that is 15,000 times that
of the Earth’s gravitational field) for 20 min, leaving a residue of cell membranes
and mitochondria. The supernatant is isolated by decanting from the residue
and is centrifuged at 30,000 ´g for 30 min, leaving a residue of lysosomes.
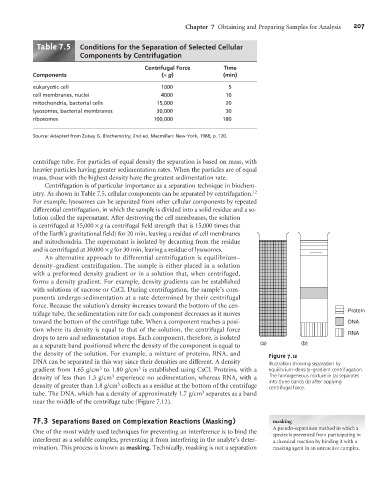
An alternative approach to differential centrifugation is equilibrium–
density–gradient centrifugation. The sample is either placed in a solution
with a preformed density gradient or in a solution that, when centrifuged,
forms a density gradient. For example, density gradients can be established
with solutions of sucrose or CsCl. During centrifugation, the sample’s com-
ponents undergo sedimentation at a rate determined by their centrifugal
force. Because the solution’s density increases toward the bottom of the cen-
Protein
trifuge tube, the sedimentation rate for each component decreases as it moves
toward the bottom of the centrifuge tube. When a component reaches a posi- DNA
tion where its density is equal to that of the solution, the centrifugal force
RNA
drops to zero and sedimentation stops. Each component, therefore, is isolated
as a separate band positioned where the density of the component is equal to (a) (b)
the density of the solution. For example, a mixture of proteins, RNA, and Figure 7.12
DNA can be separated in this way since their densities are different. A density Illustration showing separation by
3
3
gradient from 1.65 g/cm to 1.80 g/cm is established using CsCl. Proteins, with a equilibrium–density–gradient centrifugation.
3
density of less than 1.3 g/cm experience no sedimentation, whereas RNA, with a The homogeneous mixture in (a) separates
into three bands (b) after applying
3
density of greater than 1.8 g/cm collects as a residue at the bottom of the centrifuge centrifugal force.
3
tube. The DNA, which has a density of approximately 1.7 g/cm separates as a band
near the middle of the centrifuge tube (Figure 7.12).
7 3 Separations Based on Complexation Reactions (Masking) masking
F.
A pseudo-separation method in which a
One of the most widely used techniques for preventing an interference is to bind the
species is prevented from participating in
interferent as a soluble complex, preventing it from interfering in the analyte’s deter- a chemical reaction by binding it with a
mination. This process is known as masking. Technically, masking is not a separation masking agent in an unreactive complex.