Page 226 - Modern Analytical Chemistry
P. 226
1400-CH07 9/8/99 4:03 PM Page 209
Chapter 7 Obtaining and Preparing Samples for Analysis 209
2–
Ni(CN) 4 is greater than that for the Ni–EDTA complex. In fact, the
equilibrium constant for the reaction in which EDTA displaces the masking
agent
2–
4–
2–
Ni(CN) 4 +Y t NiY + 4CN –
K f 42 . ´10 18 - 12
K = = 30 = 25 . ´10
b 4 17 . ´10
2–
is very small, indicating that Ni(CN) 4 is relatively inert in the presence of
EDTA.
7F.4 Separations Based on a Change of State
Since an analyte and interferent are usually in the same phase, a separation often
can be effected by inducing a change in one of their physical or chemical states.
Changes in physical state that have been exploited for the purpose of a separation
include liquid-to-gas and solid-to-gas phase transitions. Changes in chemical state
involve one or more chemical reactions.
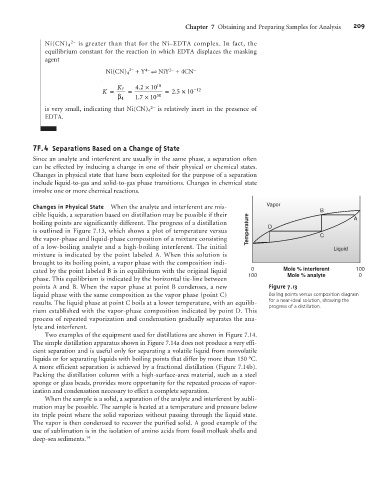
Changes in Physical State When the analyte and interferent are mis- Vapor
B
cible liquids, a separation based on distillation may be possible if their A
boiling points are significantly different. The progress of a distillation D
is outlined in Figure 7.13, which shows a plot of temperature versus Temperature C
the vapor-phase and liquid-phase composition of a mixture consisting
of a low-boiling analyte and a high-boiling interferent. The initial Liquid
mixture is indicated by the point labeled A. When this solution is
brought to its boiling point, a vapor phase with the composition indi-
cated by the point labeled B is in equilibrium with the original liquid 0 Mole % interferent 100
100 Mole % analyte 0
phase. This equilibrium is indicated by the horizontal tie-line between
points A and B. When the vapor phase at point B condenses, a new Figure 7.13
liquid phase with the same composition as the vapor phase (point C) Boiling points versus composition diagram
results. The liquid phase at point C boils at a lower temperature, with an equilib- for a near-ideal solution, showing the
progress of a distillation.
rium established with the vapor-phase composition indicated by point D. This
process of repeated vaporization and condensation gradually separates the ana-
lyte and interferent.
Two examples of the equipment used for distillations are shown in Figure 7.14.
The simple distillation apparatus shown in Figure 7.14a does not produce a very effi-
cient separation and is useful only for separating a volatile liquid from nonvolatile
liquids or for separating liquids with boiling points that differ by more than 150 °C.
A more efficient separation is achieved by a fractional distillation (Figure 7.14b).
Packing the distillation column with a high-surface-area material, such as a steel
sponge or glass beads, provides more opportunity for the repeated process of vapor-
ization and condensation necessary to effect a complete separation.
When the sample is a solid, a separation of the analyte and interferent by subli-
mation may be possible. The sample is heated at a temperature and pressure below
its triple point where the solid vaporizes without passing through the liquid state.
The vapor is then condensed to recover the purified solid. A good example of the
use of sublimation is in the isolation of amino acids from fossil mollusk shells and
deep-sea sediments. 14